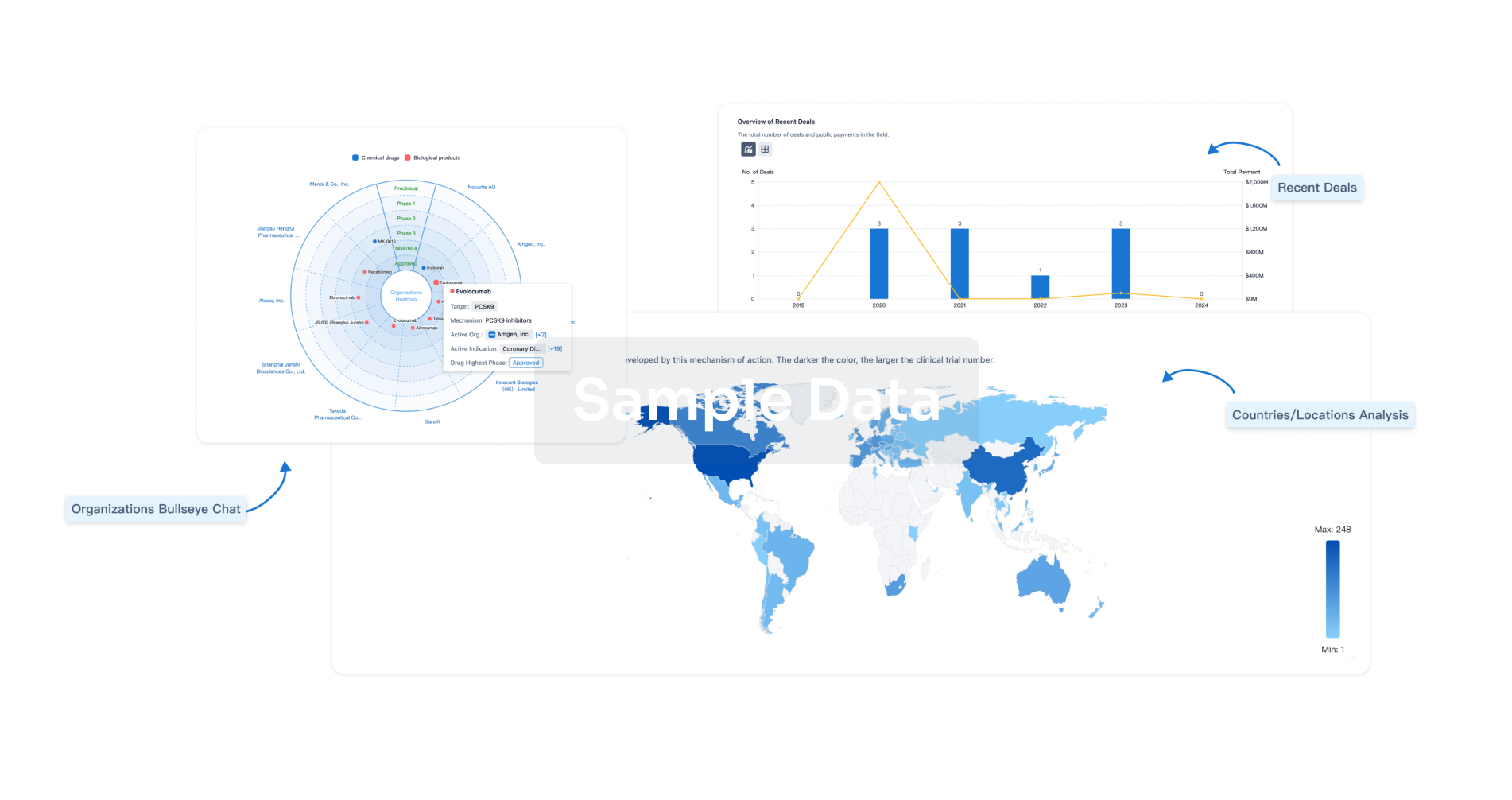
|
|
|
|
|
|
Drug Highest PhasePhase 2 |
First Approval Ctry. / Loc.- |
First Approval Date20 Jan 1800 |
/ Not yet recruitingNot Applicable An open label, balanced, randomized, two-treatment, two period, two-sequence, single oral dose, crossover, bioequivalence study of Nitrocontin Controlled Release tablet 2.6 mg (Nitroglycerin) of Modi-Mundi Pharma Pvt. Ltd., in comparison with Nitromint® Retard Controlled Release tablet 2.6 mg (Nitroglycerin) of M/s Egis Pharma, Hungary in healthy, adult, human subjects under fasting condition. - NIL
A single site, open label study to evaluate the pharmacodynamic effects of a single dose of oral anatabine citrate in healthy adult volunteers.
An Open-Label, Randomized, Single Dose, Four-way Crossover, Multi-stage Study to Determine the Comparative Bioavailability of Two Fixed Dose Combination Tablet Formulations, 500 mg or 1000 mg Extended Release Metformin and 1 mg or 2 mg Extended Release Glimepiride, in Healthy Adult Male and Female Subjects in the Fed State
This is a an open-label, randomized, single dose, four-way crossover, multi-stage study enrolling 20 healthy adult male and female subjects per part. This study consists of two separate parts (Part A and B) with each part comprising four treatment periods. Each subject will participate in all four treatment periods per part; Subjects may not enrol in both Parts A and B.
This study is being conducted to compare the pharmacokinetics (PK) of two extended release fixed dose combinations (FDC) oral formulations of metformin and glimepiride at two doses, 500mg/1mg and 1000mg/2mg, with each FDC formulation to be administered orally as a single dose and compared with the commercially available formulations of metformin extended release (XR) (GLUCOPHAGE ™ Sustained Release [SR]) and glimepiride immediate release (IR) (AMARYL ™).
Part A of study will evaluate the bioavailability of a formulation comprising a film coated tablet containing release controlling polymers; and Part B will evaluate the bioavailability of a formulation comprising a tablet coated with release controlling polymers.
In each part there will be 4 treatment periods. During each period, subjects will be randomized sequentially to receive a single dose of a reference treatment of 500 mg metformin XR / 1 mg glimepiride IR; and a reference treatment of 1000 mg metformin XR / 2 mg glimepiride IR; and an FDC tablet containing 500 mg metformin XR and 1 mg glimepiride XR; and an FDC tablet containing 1000 mg metformin XR and 2 mg glimepiride XR.Serial PK sampling for up to 36 hours and safety assessments will be performed. Each period will be separated by a washout period of at least 5 days and a follow-up visit will occur 14 days after the last dose of study drug.
100 Clinical Results associated with STAT3 x NF-κB x nAChRα1/β1/δ/γ x APP x GSK-3β
100 Translational Medicine associated with STAT3 x NF-κB x nAChRα1/β1/δ/γ x APP x GSK-3β
0 Patents (Medical) associated with STAT3 x NF-κB x nAChRα1/β1/δ/γ x APP x GSK-3β