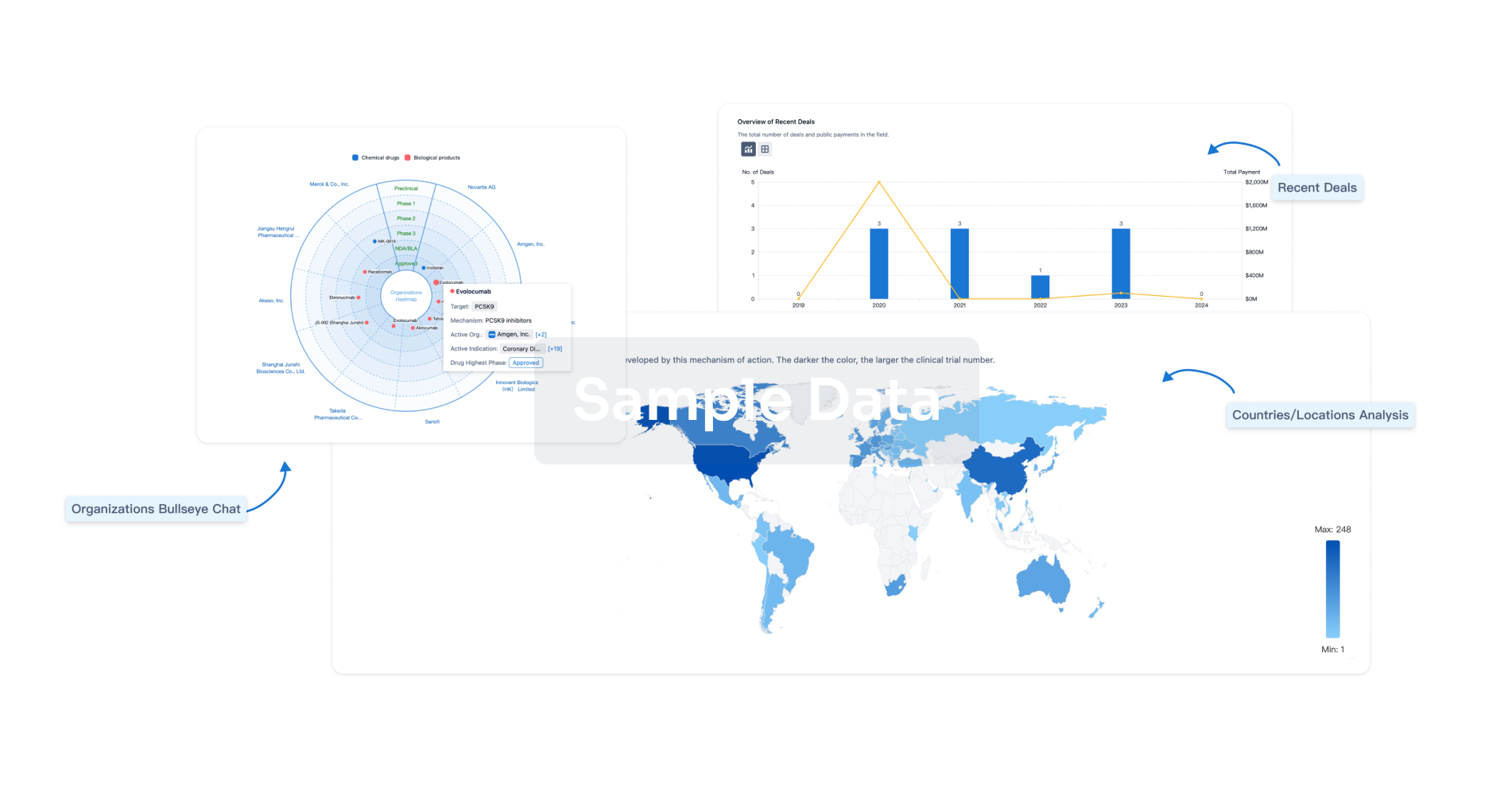
|
|
|
|
|
|
Drug Highest PhasePhase 1 |
First Approval Ctry. / Loc.- |
First Approval Date20 Jan 1800 |
PNOC023: Open Label Phase 1 and Target Validation Study of ONC206 in Children and Young Adults With Newly Diagnosed or Recurrent Diffuse Midline Glioma (DMG), and Other Recurrent Primary Malignant Central Nervous System (CNS) Tumors
This phase I trial studies the effects and best dose of ONC206 alone or in combination with radiation therapy in treating patients with diffuse midline gliomas that is newly diagnosed or has come back (recurrent) or other recurrent primary malignant CNS tumors. ONC206 is a recently discovered compound that may stop cancer cells from growing. This drug has been shown in laboratory experiments to kill brain tumor cells by causing a so called "stress response" in tumor cells. This stress response causes cancer cells to die, but without affecting normal cells. ONC206 alone or in combination with radiation therapy may be effective in treating newly diagnosed or recurrent diffuse midline gliomas and other recurrent primary malignant CNS tumors.
A First-in-human Phase I Single-agent Dose-escalation, Food Effect and Dose Expansion Study of Oral ONC206 in Recurrent and Rare Primary Central Nervous System Neoplasms
The primary objective of this Phase 1, open-label, dose-escalation, and exploratory study is to evaluate the safety and tolerability profile (establish the maximum-tolerated dose) and evaluate the occurrence of dose-limiting toxicities (DLTs) following single weekly or multiple-day weekly dose regimens of single-agent, oral ONC206 in patients with recurrent, primary central nervous system (CNS) neoplasms.
100 Clinical Results associated with CLPP x D3 receptor x D2 receptor
100 Translational Medicine associated with CLPP x D3 receptor x D2 receptor
0 Patents (Medical) associated with CLPP x D3 receptor x D2 receptor