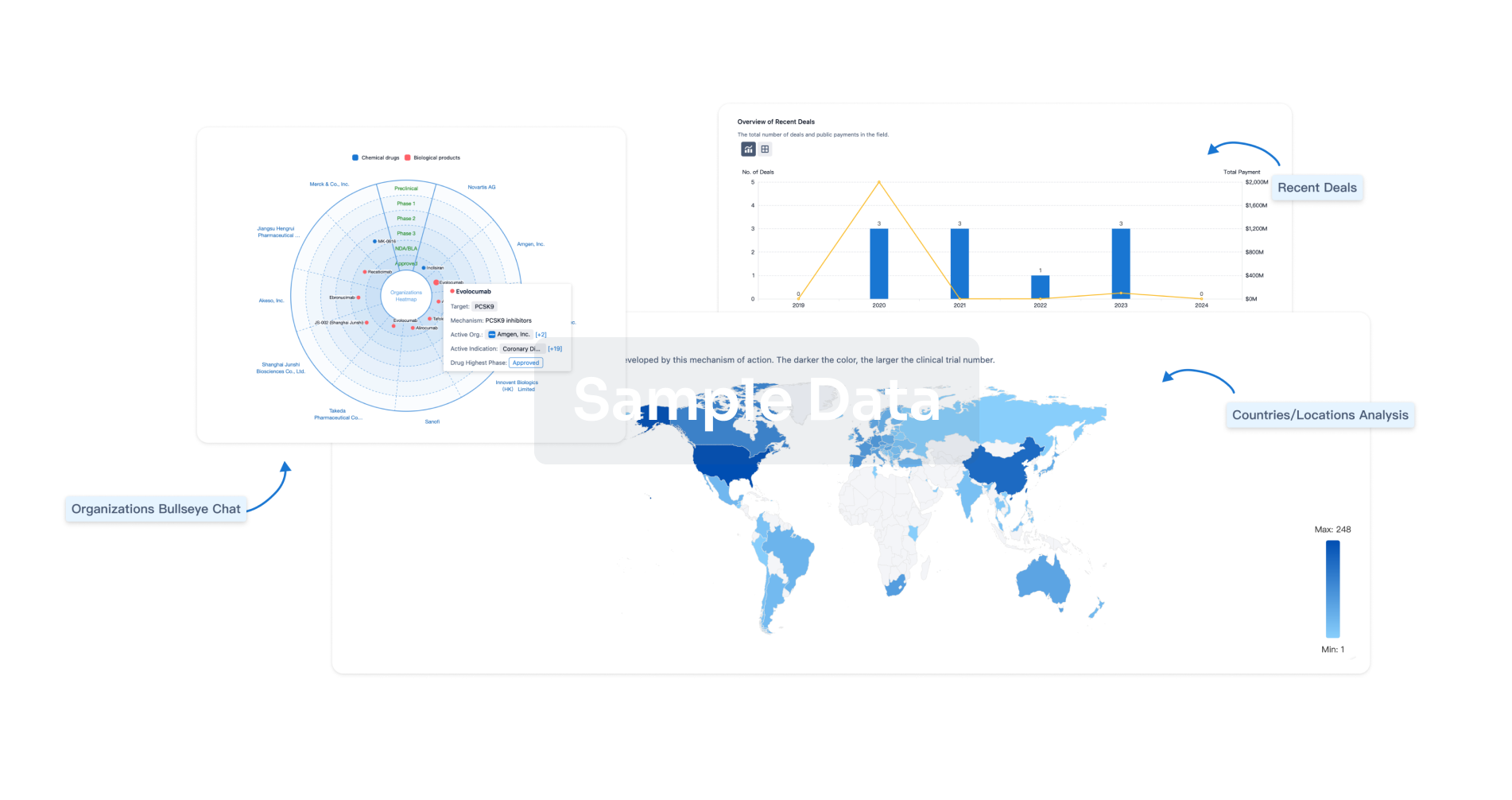
|
|
|
|
|
|
Drug Highest PhaseApproved |
First Approval Ctry. / Loc.United States |
First Approval Date17 Nov 1995 |
/ Not yet recruitingPhase 2IIT Efficacy and Safety of Liposomal Doxorubicin Combined with Vinorelbine in the Treatment of Advanced Metastatic Breast Cancer: a Single-Center, Prospective Phase II Clinical Study
With advancements in various treatment modalities, the survival of breast cancer patients has continuously improved. Patients with advanced breast cancer who have undergone multiple lines of therapy still have treatment options, but standard treatment protocols are lacking. Anthracyclines are a cornerstone in breast cancer treatment; however, their cumulative dose-related cardiac toxicity limits their use. Liposomal doxorubicin exhibits comparable efficacy to conventional anthracyclines and is not affected by previous cumulative doses. The combination of vinorelbine with liposomal doxorubicin shows reduced cross-toxicity, and several studies have demonstrated the effectiveness of this regimen in metastatic HER2-negative breast cancer patients.
Therefore, we aim to explore whether optimizing the dosage and treatment cycle of this combination therapy can provide a viable treatment option for metastatic HER2-negative breast cancer patients who have previously received second-line or higher chemotherapy, seeking a regimen that balances efficacy and safety.
This study is a single-center, single-arm Phase II clinical trial planned to enroll 30 metastatic HER2-negative breast cancer patients who have previously undergone second-line or higher chemotherapy. Participants will receive an optimized regimen of liposomal doxorubicin combined with vinorelbine, with safety assessed every cycle and efficacy evaluated every three cycles. Treatment will continue until radiographic evidence indicates disease progression, intolerable toxicity occurs, informed consent is withdrawn, or the investigator decides to discontinue treatment. Following treatment, each participant will undergo survival follow-up every three months until death, loss to follow-up, or withdrawal of consent.
/ Not yet recruitingPhase 4 A Multicenter, Single-arm, Open-label, Phase 4 Study to Evaluate the Safety and Efficacy of Brentuximab Vedotin plus Doxorubicin, Vinblastine and Dacarbazine in Indian Patients with Untreated Stage 3/4 Classical Hodgkin Lymphoma - NIL
100 Clinical Results associated with Reactive oxygen species x Top II x DNA
100 Translational Medicine associated with Reactive oxygen species x Top II x DNA
0 Patents (Medical) associated with Reactive oxygen species x Top II x DNA