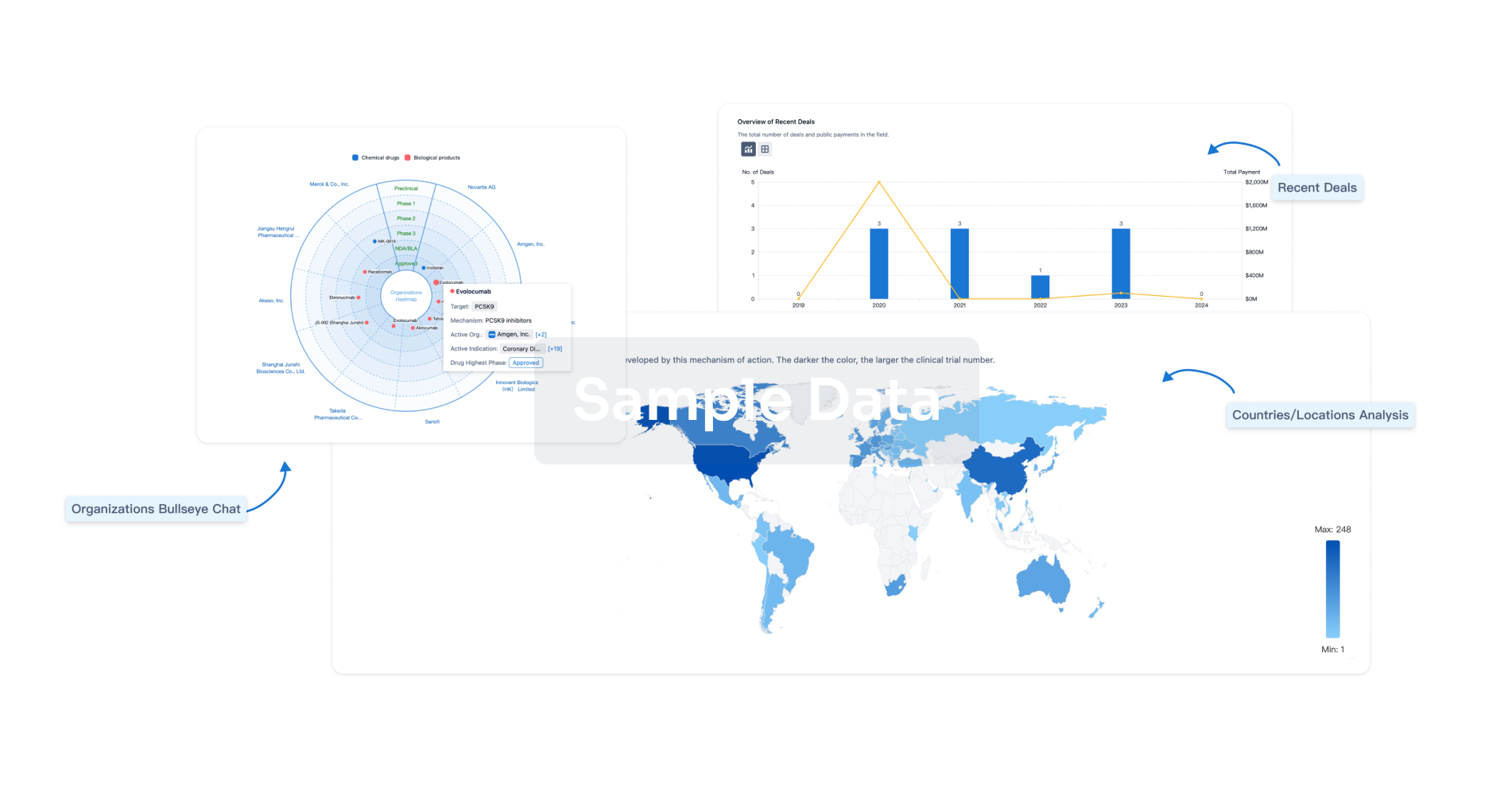
|
|
Active Org.- |
|
Active Indication- |
|
Drug Highest PhasePending |
First Approval Ctry. / Loc.- |
First Approval Date20 Jan 1800 |
Phase 2 Trial of BMS-690514 in Non-Small Cell Lung Cancer Subjects Who Have Been Treated With Gefitinib or Erlotinib and Are Genotypically EGFR Mutation Positive or Who Have Had a Prior Response
Start Date01 Sep 2010 |
Sponsor / Collaborator- |
Phase 2 Trial of BMS-690514 in Non-Small Cell Lung Cancer Subjects Who Have Been Treated With Gefitinib or Erlotinib and Are Genotypically EGFR Mutation Positive or Who Have Had a Prior Response
The purpose of this study is to observe an improvement in overall response rate in NSCLC subjects who have been treated with gefitinib or erlotinib and are genotypically EGFR mutation positive or who have had a prior a response.
An Open-Label Randomized, Parallel, Two-Arm Phase II Study Comparing BMS-690514 + Letrozole With Lapatinib + Letrozole in Recurrent and Metastatic Breast Cancer Patients Who Are Hormone Receptor Positive Despite HER2 Status And Who Relapsed While Receiving or After Completing Adjuvant Antiendocrine Therapy
The purpose of this study is to determine if BMS-690514 + letrozole will be more effective than lapatinib + letrozole in patients who have metastatic hormone receptor positive breast cancer after developing progressive disease immediately following adjuvant antiendocrine therapy
100 Clinical Results associated with HER2 x VEGFR1 x VEGFR2 x HER4 x VEGFR3 x EGFR x VEGFR
100 Translational Medicine associated with HER2 x VEGFR1 x VEGFR2 x HER4 x VEGFR3 x EGFR x VEGFR
0 Patents (Medical) associated with HER2 x VEGFR1 x VEGFR2 x HER4 x VEGFR3 x EGFR x VEGFR