Last update 16 May 2024
Topotecan Hydrochloride
Last update 16 May 2024
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms 9-[(dimethylamino)methyl]-10-hydroxy-(4S)-camptothecin, Nogitecan hydrochloride (JAN), Topotecan hydrochloride (USAN) + [21] |
Target |
Mechanism TOP1 inhibitors(DNA topoisomerase I inhibitors) |
Therapeutic Areas |
Active Indication |
Inactive Indication |
Originator Organization |
Active Organization |
Inactive Organization |
Drug Highest PhaseApproved |
First Approval Date US (28 May 1996), |
RegulationOrphan Drug (EU) |
Login to view First Approval Timeline
Structure
Molecular FormulaC23H23N3O5 |
InChIKeyUCFGDBYHRUNTLO-QHCPKHFHSA-N |
CAS Registry123948-87-8 |
View All Structures (2)
Related
377
Clinical Trials associated with Topotecan HydrochlorideA Phase 3 Study of Dinutuximab Added to Intensive Multimodal Therapy for Children With Newly Diagnosed High-Risk Neuroblastoma
This phase III trial tests how well adding dinutuximab to induction chemotherapy along with standard of care surgery radiation and stem cell transplantation works for treating children with newly diagnosed high risk neuroblastoma. Dinutuximab is a monoclonal antibody that binds to a molecule called GD2, which is found in greater than normal amounts on some types of cancer cells. This helps cells of the immune system kill the cancer cells. Chemotherapy drugs such as cyclophosphamide, topotecan, cisplatin, etoposide, vincristine, dexrazoxane, doxorubicin, temozolomide, irinotecan and isotretinoin, work in different ways to stop the growth of cancer cells, either by killing the cells, by stopping them from dividing or by stopping them from spreading. During induction, chemotherapy and surgery are used to kill and remove as much tumor as possible. During consolidation, very high doses of chemotherapy are given to kill any remaining cancer cells. This chemotherapy also destroys healthy bone marrow, where blood cells are made. A stem cell transplant is a procedure that helps the body make new healthy blood cells to replace the blood cells that may have been harmed by the cancer and/or chemotherapy. Radiation therapy is also given to the site where the cancer originated (primary site) and to any other areas that are still active at the end of induction.
Start Date19 Apr 2024 |
Sponsor / Collaborator |
DAREON™-9: A Phase Ib Open-label Dose Escalation and Dose Confirmation Safety Study of Intravenous BI 764532 in Combination With Topotecan for the Treatment of Patients With Small Cell Lung Cancer
This study is open to adults with extensive stage small cell lung cancer. The study is in people with advanced cancer that had previously received platinum-based chemotherapy and are eligible to receive topotecan treatment.
The purpose of this study is to find out the highest dose of BI 764532 that people can tolerate when taken together with topotecan. BI 764532 is an antibody-like molecule that may help the immune system fight cancer. Participants get BI 764532 and topotecan as infusions into a vein. As an alternative, topotecan may also be taken orally (tablets).
Participants may continue to take BI 764532 as long as they benefit from treatment and can tolerate it. During this time, participants visit the study site regularly. The visits also depend on the response to the treatment. At the study visits, the doctors check the health of the participants, take necessary laboratory tests, and note any health problems that could have been caused by the study treatment.
The purpose of this study is to find out the highest dose of BI 764532 that people can tolerate when taken together with topotecan. BI 764532 is an antibody-like molecule that may help the immune system fight cancer. Participants get BI 764532 and topotecan as infusions into a vein. As an alternative, topotecan may also be taken orally (tablets).
Participants may continue to take BI 764532 as long as they benefit from treatment and can tolerate it. During this time, participants visit the study site regularly. The visits also depend on the response to the treatment. At the study visits, the doctors check the health of the participants, take necessary laboratory tests, and note any health problems that could have been caused by the study treatment.
Start Date15 Jan 2024 |
Sponsor / Collaborator |
A Randomized, Double-Blind, Placebo-Controlled Study of Trilaciclib vs Placebo in Patients With Extensive Stage Small Cell Lung Cancer (ES-SCLC) Receiving Topotecan Chemotherapy
This is a multicenter, randomized, double-blind, placebo-controlled study to assess whether trilaciclib administered prior to topotecan is non-inferior to placebo administered prior to topotecan with regard to overall survival.
Start Date18 Oct 2023 |
Sponsor / Collaborator |
100 Clinical Results associated with Topotecan Hydrochloride
Login to view more data
100 Translational Medicine associated with Topotecan Hydrochloride
Login to view more data
100 Patents (Medical) associated with Topotecan Hydrochloride
Login to view more data
3,266
Literatures (Medical) associated with Topotecan Hydrochloride01 Dec 2024·Molecular Biology Reports
Synergistic effect of a nonsteroidal anti-inflammatory drug in combination with topotecan on small cell lung cancer cells
Article
Author: Yanar, Sevinc ; Bal Albayrak, Merve Gulsen ; Eskiler, Gamze Guney ; Kasap, Murat ; Kanli, Aylin ; Ozkan, Asuman Deveci
01 Jun 2024·Translational Oncology
Development of a novel prostate Cancer-Stroma Sphere (CSS) model for In Vitro tumor microenvironment studies
Article
Author: Zolotykh, Maria A ; Mingaleeva, Rimma N ; Rakhmatullina, Aigul R ; Filina, Yulia V ; Gafurbaeva, Dina U ; Sagdeeva, Aisylu R ; Rizvanov, Albert A ; Bulatov, Emil R ; Miftakhova, Regina R ; Boulygina, Eugenia A
01 Jun 2024·Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Effect of the combined binding of topotecan and catechin/protocatechuic acid to a pH-sensitive DNA tetrahedron on release and cytotoxicity: Spectroscopic and calorimetric studies
Article
Author: Zhang, Xinpeng ; Liu, Jie ; Li, Xinyu ; Wang, Lu ; Yuan, Lixia ; Wang, Xiangtai ; Wu, Yushu ; Liu, Min
123
News (Medical) associated with Topotecan Hydrochloride01 May 2024
Achieved $14.1 Million in Net Revenue from Sales of COSELA® (trilaciclib) for First Quarter 2024
Reaffirmed 2024 Net COSELA Revenue Guidance of $60 to $70 Million
Announced That Updated Efficacy Results from Phase 2 Trial of Trilaciclib in Combination with a TROP2 Antibody-Drug Conjugate (ADC) Will Be Presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting
Announced That Final Analysis of Phase 3 PRESERVE 2 Trial Evaluating Overall Survival in 1L Metastatic Triple Negative Breast Cancer (mTNBC) Is Expected to Occur in Late Second Quarter of 2024
Cash Runway Expected to Extend into the Third Quarter of 2025
Management to Host Webcast and Conference Call today at 8:30 AM ET
RESEARCH TRIANGLE PARK, N.C., May 01, 2024 (GLOBE NEWSWIRE) -- G1 Therapeutics, Inc. (Nasdaq: GTHX), a commercial-stage oncology company, today provided a corporate and financial update for the first quarter ended March 31, 2024.
“Our focus for 2024 is on developing trilaciclib toward potential category leadership in triple negative breast cancer and maximizing the uptake of COSELA in its first indication in extensive stage small cell lung cancer; we've made progress on both in the first four months of the year,” said Jack Bailey, Chief Executive Officer of G1 Therapeutics. “Regarding our clinical progress, we have two important readouts later this year, the first of which is an ASCO poster presentation in early June on the mature results from our Phase 2 trial of trilaciclib in combination with a TROP2 ADC. This will be followed by the readout of our Phase 3 PRESERVE 2 1L mTNBC trial late in the second quarter of this year. Regarding COSELA for extensive stage small cell lung cancer, we remain confident in our annual net sales guidance of $60 to $70 million."
First Quarter 2024 and Recent Highlights
Financial
Recognized $14.1 Million in Net COSELA Revenue: Vial volume grew four percent in the first quarter of 2024 over the prior quarter.
Cash Runway Extends into the Third Quarter of 2025: G1 ended the first quarter of 2024 with cash, cash equivalents, and marketable securities of $65.2 million.
Clinical
Updated Efficacy Results from Phase 2 Trial of Trilaciclib in Combination with an ADC Accepted for Poster Presentation at the 2024 ASCO Annual Meeting: In January 2024, the Company provided preliminary data from this Phase 2 trial in combination with the TROP2 ADC sacituzumab govitecan (SG) in patients with mTNBC suggesting clinically meaningful improvements in overall survival among patients receiving trilaciclib with SG compared to SG alone using historical data from the ASCENT study. Updated efficacy results will be presented in a poster at the 2024 ASCO Meeting, which is being held from May 31 to June 4, 2024.
Final Analysis of the Phase 3 PRESERVE 2 Trial in 1L mTNBC is Estimated to Occur in Late Second Quarter 2024: The final analysis will be conducted on the intent-to-treat (ITT) population, which includes the survival events from patients enrolled in the Ukraine. Based on recent interactions with the U.S. Food and Drug Administration regarding the inclusion of these events, the final analysis is now expected late in the second quarter of 2024. If the pivotal results are positive, the Company will engage the U.S. Food and Drug Administration ahead of a supplemental New Drug Application (sNDA) filing for this indication.
Corporate
Announced License Agreement for Lerociclib with Pepper Bio, Inc. (“Pepper Bio”): Pepper Bio will gain exclusive rights to develop, manufacture, and commercialize lerociclib for all indications except for certain radioprotectant uses in the US, Europe, Japan, and all other global markets, excluding the Asia-Pacific region. Under the terms of the agreement, G1 is expected to receive upfront payments totaling mid-single digit millions within 12 months and is eligible to receive a maximum of $135M upon achievement of development and commercial milestones in up to three indications. In addition, Pepper Bio will pay G1 a double-digit royalty on aggregate annual net sales of lerociclib. (See May 1, 2024 press release here)
First Quarter 2024 Financial Results
As of March 31, 2024, cash, cash equivalents and marketable securities totaled $65.2 million, compared to $82.2 million as of December 31, 2023. The Company believes that its current cash runway is sufficient to fund its operations into the third quarter of 2025.
Total revenues for the first quarter of 2024 were $14.5 million, including $14.1 million in net product sales of COSELA and license revenue of $0.4 million, related to patent and clinical trial costs reimbursed by EQRx, Simcere, and Genor, compared to $12.9 million in total revenues in the first quarter of 2023.
Operating expenses for the first quarter of 2024 were $23.5 million, compared to $38.7 million for the first quarter of 2023. GAAP operating expenses include stock-based compensation expense of $2.5 million for the first quarter of 2024, compared to $3.8 million for the first quarter of 2023.
Cost of goods sold expense for the first quarter of 2024 was $1.1 million, compared to $1.5 million for the first quarter of 2023, the decrease was primarily due to a cancellation fee recognized during the quarter ended March 31, 2023.
Research and development (R&D) expenses for the first quarter of 2024 were $7.3 million, compared to $15.5 million for the first quarter of 2023. The decrease in R&D expenses was primarily due to a decrease in the Company's clinical program costs.
Selling, general, and administrative (SG&A) expenses for the first quarter of 2024 were $15.1 million, compared to $21.8 million for the first quarter of 2023. The decrease in SG&A expenses was primarily due to decreases in personnel costs, commercialization activities, and medical affairs.
The net loss for the first quarter of 2024 was $10.2 million, compared to $27.6 million for the first quarter of 2023. The basic and diluted net loss per share for the first quarter of 2024 was $(0.20), compared to $(0.53) for the first quarter of 2023.
2024 Financial Guidance
G1 today reaffirmed its full year 2024 financial guidance. The Company expects to generate between $60 million and $70 million in COSELA net revenue in 2024. G1's product revenue guidance is based on expectations for continued acceleration of sales performance of COSELA in the U.S. Additionally, the Company believes that its current cash runway is sufficient to fund its operations into the third quarter of 2025.
Webcast and Conference Call
G1 will host a webcast and conference call at 8:30 a.m. ET today to provide a corporate and financial update for the first quarter ended March 31, 2024.
Please note the following process to access the call via telephone: To register and receive a dial in number and unique PIN to access the live conference call, please follow this linkto register online. While not required, it is recommended to join 10 minutes prior to the start of the event. A live and archived webcast will be available on the Events & Presentations page of the Company’s website: The webcast will be archived on the same page for 90 days following the event.
About COSELA® (trilaciclib) for Injection
COSELA (trilaciclib) was approved by the U.S. Food and Drug Administration on February 12, 2021.
Indication
COSELA® (trilaciclib) is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer.
Important Safety Information
COSELA is contraindicated in patients with a history of serious hypersensitivity reactions to trilaciclib.
Warnings and precautions include injection-site reactions (including phlebitis and thrombophlebitis), acute drug hypersensitivity reactions, interstitial lung disease (pneumonitis), and embryo-fetal toxicity.
The most common adverse reactions (>10%) were fatigue, hypocalcemia, hypokalemia, hypophosphatemia, aspartate aminotransferase increased, headache, and pneumonia.
This information is not comprehensive. Please click here for full Prescribing Information.
To report suspected adverse reactions, contact G1 Therapeutics at 1-800-790-G1TX or call FDA at 1-800-FDA-1088 or visit
About G1 Therapeutics
G1 Therapeutics, Inc. is a commercial-stage oncology biopharmaceutical company whose mission is to develop and deliver next-generation therapies that improve the lives of those affected by cancer, including the Company’s first commercial product, COSELA® (trilaciclib). The Company is also evaluating therapies in combination with cytotoxic therapies and/or immunotherapy in areas of high unmet need including triple-negative breast cancer and extensive stage small cell lung cancer. G1’s goal is to provide innovative therapeutic advances for people living with cancer. For additional information, please visit and follow us on X (formerly known as Twitter) @G1Therapeutics and LinkedIn.
G1 Therapeutics® and the G1 Therapeutics logo and COSELA® and the COSELA logo are trademarks of G1 Therapeutics, Inc.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "plan," "anticipate," “could”, “believe,” “goal”, “projections,” "estimate," "intend," “indicate,” “potential,” “opportunity,” “suggest,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Forward-looking statements in this press release include, but are not limited to, those related to the timing of results from G1’s ongoing clinical trials and developing trilaciclib toward potential category leadership in triple negative breast cancer and maximizing the uptake of COSELA in its first indication in extensive stage small cell lung cancer, are based on the company’s expectations and assumptions as of the date of this press release. Each of these forward-looking statements involves risks and uncertainties. Factors that may cause the company’s actual results to differ from those expressed or implied in the forward-looking statements in this press release are discussed in the company’s filings with the U.S. Securities and Exchange Commission, including the "Risk Factors" sections contained therein and include, but are not limited to, the Company’s dependence on the commercial success of COSELA (trilaciclib); the development and commercialization of new drug products is highly competitive; the Company’s ability to complete clinical trials for, obtain approvals for and commercialize any of its product candidates; the Company’s initial success in ongoing clinical trials may not be indicative of results obtained when these trials are completed or in later stage trials; the inherent uncertainties associated with developing new products or technologies and operating as a commercial-stage company; chemotherapy shortages and market conditions. Our business is subject to substantial risks and uncertainties, including those referenced above. Investors, potential investors, and others should give careful consideration to these risks and uncertainties. Except as required by law, the company assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available.
G1 Therapeutics Contacts:
John W. Umstead V
Chief Financial Officer
919-747-8419
jumstead@g1therapeutics.com
Will Roberts
Communications Officer
Vice President, Investor Relations & Corporate Communications
919-907-1944
wroberts@g1therapeutics.com
G1 Therapeutics, Inc.
Balance Sheet Data
(in thousands)
March 31, 2024
December 31, 2023
Cash and cash equivalents and Marketable securities
$
65,186
$
82,156
Working Capital
$
63,236
$
85,232
Total Assets
$
102,026
$
121,540
Accumulated deficit
$
(790,204
)
$
(779,985
)
Total stockholders' equity
$
27,739
$
35,386
G1 Therapeutics, Inc.
Condensed Statements of Operations
(in thousands, except share and per share amounts)
Three Months Ended March 31,
2024
2023
Revenues
(unaudited)
Product sales, net
$
14,079
$
10,492
License revenue
397
2,454
Total revenues
14,476
12,946
Operating expenses
Cost of goods sold
1,079
1,459
Research and development
7,318
15,480
Selling, general and administrative
15,127
21,753
Total operating expenses
23,524
38,692
Loss from operations
(9,048
)
(25,746
)
Other income (expense)
Interest income
281
716
Interest expense
(1,978
)
(3,089
)
Other income (expense)
526
524
Total other income (expense), net
(1,171
)
(1,849
)
Loss before income taxes
(10,219
)
(27,595
)
Income tax expense
—
—
Net loss
$
(10,219
)
$
(27,595
)
Net loss per share, basic and diluted
$
(0.20
)
$
(0.53
)
Weighted average common shares outstanding, basic and diluted
52,171,684
51,647,934
License out/inPhase 2Clinical ResultDrug ApprovalFinancial Statement
30 Apr 2024
Company Announcement
Full approval based on global Phase 3 study demonstrating overall survival benefit of TIVDAK compared to chemotherapy
TIVDAK is the first antibody-drug conjugate in this patient population to have positive overall survival data
April 30, 2024 – Genmab A/S (Nasdaq: GMAB) and Pfizer Inc. (NYSE: PFE) announced today the U.S. Food and Drug Administration (FDA) has approved the supplemental Biologics License Application (sBLA) for TIVDAK® (tisotumab vedotin-tftv) for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy. This FDA action converts the September 2021 accelerated approval of TIVDAK to a full approval. TIVDAK is the first antibody-drug conjugate (ADC) with demonstrated overall survival data to be granted full FDA approval in this patient population.
The approval is based on results from the global, randomized, Phase 3 innovaTV 301 clinical trial (NCT04697628), in which TIVDAK met its primary endpoint of overall survival (OS) in patients with previously treated recurrent or metastatic cervical cancer compared to chemotherapy. Secondary endpoints of progression-free survival (PFS) and a confirmed objective response rate (ORR) were also met. In October 2023, results from the innovaTV 301 study were initially disclosed during the Presidential Symposium at the European Society of Medical Oncology (ESMO) Congress.
“As a treating physician, it is encouraging to see overall survival data among these patients and a manageable safety profile with tisotumab vedotin,” said Brian Slomovitz, M.D., Director of Gynecologic Oncology and Co-Chair of the Cancer Research Committee at Mount Sinai Medical Center, Miami Beach. “Treatment options for patients with advanced or recurrent cervical cancer are limited. The five-year survival rate for patients who have metastatic disease at diagnosis is less than 20% in the U.S.i There is a high unmet need for more treatment options that have demonstrated survival benefit in the contemporary treatment landscape. The approval of tisotumab vedotin brings us a step closer to fulfilling that need.”
The innovaTV 301 study met its primary endpoint of OS, demonstrating a 30% reduction in the risk of death compared with chemotherapy (Hazard ratio [HR]: 0.70 [95% CI: 0.54, 0.89], two-sided p=0.0038ii). Median OS for patients treated with TIVDAK was 11.5 months [95% CI: 9.8-14.9] versus chemotherapy 9.5 months [95% CI: 7.9-10.7].
“The full FDA approval of TIVDAK represents a significant achievement for women with recurrent and metastatic cervical cancer, reinforcing TIVDAK as a treatment option that has proven to extend survival in patients whose disease has advanced after initial treatments,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. “This milestone underscores the importance of our ongoing clinical development program to assess the full potential of tisotumab vedotin as a treatment option in other indications.”
"Recurrent or metastatic cervical cancer is a particularly devastating and mostly incurable disease, and patients are in need of survival-extending treatment options,” said Chris Boshoff, M.D., Ph.D., Chief Oncology Officer, Executive Vice President at Pfizer. “Today’s full approval by the FDA reinforces the important role of TIVDAK for these patients, as the first antibody-drug conjugate with statistically significant prolonged overall survival data.”
The safety profile of TIVDAK in innovaTV 301 was consistent with its known safety profile as presented in the U.S. prescribing information which includes a BOXED WARNING for Ocular Toxicity. No new safety issues were identified. The most common (≥25%) adverse reactions, including laboratory abnormalities, in patients receiving TIVDAK were hemoglobin decreased (41%), peripheral neuropathy (38%), conjunctival adverse reactions (37%), aspartate aminotransferase increased (34%), nausea (33%), alanine aminotransferase increased (30%), fatigue (28%), sodium decreased (27%), epistaxis (26%), and constipation (25%).
The sBLA application received a Priority Review Designation, which is granted by the U.S. FDA to medicines that may offer significant advances in treatment or may provide a treatment where no adequate therapy exists.iii TIVDAK was granted accelerated approval in the U.S. by the FDA in September 2021, based on tumor response and durability of response from the innovaTV 204 pivotal Phase 2 single-arm clinical trial evaluating TIVDAK as a monotherapy in patients with previously treated recurrent or metastatic cervical cancer.
“Today marks a great day for patients, especially adults battling advanced cervical cancer,” said Tamika Felder, cervical cancer patient advocate and Founder and Chief Visionary Officer, Cervivor, Inc. “This full approval opens up new treatment paths for this patient community who have long faced limited options.”
About Cervical Cancer
Cervical cancer remains a disease with high unmet need despite advances in effective vaccination and screening practices to prevent and diagnose pre-/early-stage cancers for curative treatment. Recurrent and/or metastatic cervical cancer is a particularly devastating and mostly incurable disease; up to 15% of adults with cervical cancer present with metastatic disease at diagnosisiv,v and, for adults diagnosed at earlier stages who receive treatment, up to 61%vi will experience disease recurrence. It was estimated that in 2023, more than 13,960 new cases of invasive cervical cancer were diagnosed in the U.S. and 4,310 adults would die from the disease.vii
The innovaTV 301 trial (NCT04697628) is a global, 1:1 randomized, open-label Phase 3 trial evaluating TIVDAK® (tisotumab vedotin-tftv) versus investigator’s choice of single agent chemotherapy (topotecan, vinorelbine, gemcitabine, irinotecan or pemetrexed) in 502 patients with recurrent or metastatic cervical cancer who received one or two prior systemic regimens in the recurrent or metastatic setting.
Patients with recurrent or metastatic cervical cancer with squamous cell, adenocarcinoma or adenosquamous histology, and disease progression during or after treatment with chemotherapy doublet +/- bevacizumab and an anti-PD-(L)1 agent (if eligible) are included. The primary endpoint was overall survival. The main secondary outcomes were progression-free survival and objective response rate.
The study was conducted by Seagen, which was acquired by Pfizer in December 2023, in collaboration with Genmab, European Network of Gynaecological Oncological Trial Groups (ENGOT, study number ENGOT cx-12) and the Gynecologic Oncology Group (GOG) Foundation (study number GOG 3057), as well as other global gynecological oncology cooperative groups. For more information about the Phase 3 innovaTV 301 clinical trial and other clinical trials with tisotumab vedotin, please visit www.clinicaltrials.gov.
TIVDAK (tisotumab vedotin-tftv) is an antibody-drug conjugate (ADC) composed of Genmab’s human monoclonal antibody directed to tissue factor (TF) and Pfizer’s ADC technology that utilizes a protease-cleavable linker that covalently attaches the microtubule-disrupting agent monomethyl auristatin E (MMAE) to the antibody. Nonclinical data suggest that the anticancer activity of tisotumab vedotin-tftv is due to the binding of the ADC to TF-expressing cancer cells, followed by internalization of the ADC-TF complex and release of MMAE via proteolytic cleavage. MMAE disrupts the microtubule network of actively dividing cells, leading to cell cycle arrest and apoptotic cell death. In vitro, tisotumab vedotin-tftv also mediates antibody-dependent cellular phagocytosis and antibody-dependent cellular cytotoxicity.
Genmab is an international biotechnology company with a core purpose guiding its unstoppable team to strive towards improving the lives of patients through innovative and differentiated antibody therapeutics. For 25 years, its passionate, innovative and collaborative team has invented next-generation antibody technology platforms and leveraged translational, quantitative, and data sciences, resulting in a proprietary pipeline including bispecific T-cell engagers, next-generation immune checkpoint modulators, effector function enhanced antibodies, and antibody-drug conjugates. To help develop and deliver novel antibody therapies to patients, Genmab has formed 20+ strategic partnerships with biotechnology and pharmaceutical companies. By 2030, Genmab’s vision is to transform the lives of people with cancer and other serious diseases with knock-your-socks-off (KYSO®) antibody medicines.
At Pfizer Oncology, we are at the forefront of a new era in cancer care. Our industry-leading portfolio and extensive pipeline includes game-changing mechanisms of action to attack cancer from multiple angles, including antibody-drug conjugates (ADCs), small molecules, bispecific antibodies and other immunotherapy biologics. We are focused on delivering transformative therapies in some of the world’s most common cancers, including breast cancer, genitourinary cancer, hematology-oncology and thoracic cancers, which includes lung cancer. Driven by science, we are committed to accelerating breakthroughs to extend and improve patients’ lives.
Tisotumab vedotin is co-owned by Genmab and Pfizer, under an agreement in which the companies share costs and profits for the product on a 50:50 basis.
i Cervical Cancer: Statistics. American Society of Clinical Oncology (ASCO). September 2023. https://www.cancer.net/cancer-types/cervical-cancer/statistics
ii The threshold for statistical significance is 0.0226 (two-sided).
iii Priority Review. U.S. Food and Drug Administration. January 4, 2018. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/priority-review
iv National Cancer Institute. SEER Cancer Stat Facts: Cervical Cancer. 2023. https://seer.cancer.gov/statfacts/html/cervix.html
v McLachlan J, Boussios S, Okines A, et al. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin Oncol (R Coll Radiol). 2017;29(3):153-60.
vi Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016 Jan;214(1):22-30. doi: 10.1016/j.ajog.2015.07.022. Epub 2015 Jul 26. PMID: 26212178; PMCID: PMC5613936.
vii Key Statistics for Cervical Cancer. American Cancer Society. Atlanta, GA. 2023. https://www.cancer.org/cancer/types/cervical-cancer/about/key-statistics.html
The content above comes from the network. if any infringement, please contact us to modify.

Clinical ResultDrug ApprovalASCOPriority Review
29 Apr 2024
TIVDAK is the first antibody-drug conjugate (ADC) to have positive overall survival data for patients with previously treated recurrent or metastatic cervical cancer
Conversion to full approval from accelerated approval is based on positive results from global Phase 3 study demonstrating overall survival benefit of TIVDAK compared to chemotherapy
NEW YORK & COPENHAGEN, Denmark--(BUSINESS WIRE)-- Pfizer Inc. (NYSE: PFE) and Genmab A/S (Nasdaq: GMAB) today announced the U.S. Food and Drug Administration (FDA) approves the supplemental Biologics License Application (sBLA) granting full approval for TIVDAK® (tisotumab vedotin-tftv) for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy.
"Recurrent or metastatic cervical cancer is a particularly devastating and mostly incurable disease, and patients are in need of survival-extending treatment options,” said Chris Boshoff, M.D., Ph.D., Chief Oncology Officer, Executive Vice President at Pfizer. “Today’s full approval by the FDA reinforces the important role of TIVDAK for these patients, as the first antibody-drug conjugate with statistically significant prolonged overall survival data.”
The approval is based on results from the global, randomized, Phase 3 innovaTV 301 clinical trial (NCT04697628), which met its primary endpoint, demonstrating overall survival (OS) benefit in adult patients with previously treated recurrent or metastatic cervical cancer treated with TIVDAK compared to chemotherapy. Secondary endpoints of progression-free survival (PFS) and confirmed objective response rate (ORR) were also met. In October 2023, results from the innovaTV 301 study were presented during the Presidential session at the European Society of Medical Oncology (ESMO) Congress.
The innovaTV 301 study demonstrated a 30% reduction in the risk of death compared to chemotherapy (hazard ratio [HR]: 0.70 [95% CI: 0.54-0.89], two-sided p=0.0038)i. Median OS for patients treated with TIVDAK was 11.5 months [95% CI: 9.8-14.9] versus chemotherapy 9.5 months [95% CI: 7.9-10.7].
“The full FDA approval of TIVDAK represents a significant achievement for women with recurrent and metastatic cervical cancer, reinforcing TIVDAK as a treatment option that has proven to extend survival in patients whose disease has advanced after initial treatments,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. “This milestone underscores the importance of our ongoing clinical development program to assess the full potential of tisotumab vedotin as a treatment option in other indications.”
The U.S. Prescribing Information for TIVDAK includes a BOXED WARNING for Ocular Toxicity as well as the following Warnings and Precautions: peripheral neuropathy, hemorrhage, pneumonitis, severe cutaneous adverse reactions, and embryo-fetal toxicity. Please see below for additional Important Safety Information.
The safety pro TIVDAK in innovaTV 301 was consistent with its known safety pro presented in the U.S. prescribing information. No new safety issues were identified. The most common (≥25%) adverse reactions, including laboratory abnormalities, in patients receiving TIVDAK were hemoglobin decreased (41%), peripheral neuropathy (38%), conjunctival adverse reactions (37%), aspartate aminotransferase increased (34%), nausea (33%), alanine aminotransferase increased (30%), fatigue (28%), sodium decreased (27%), epistaxis (26%), and constipation (25%).
“As a treating physician, it is encouraging to see overall survival data among these patients and a manageable safety pro tisotumab vedotin,” said Brian Slomovitz, M.D., Director of Gynecologic Oncology and Co-Chair of the Cancer Research Committee at Mount Sinai Medical Center, Miami Beach. “Treatment options for patients with advanced or recurrent cervical cancer are limited. The five-year survival rate for patients who have metastatic disease at diagnosis is less than 20% in the U.S.ii There is a high unmet need for more treatment options that have demonstrated survival benefit in the contemporary treatment landscape. The approval of tisotumab vedotin brings us a step closer to fulfilling that need.”
The sBLA application received a Priority Review Designation, which is granted by the FDA to medicines that may offer significant advances in treatment or may provide a treatment where no adequate therapy exists.iii TIVDAK was originally granted accelerated approval in the U.S. by the FDA in September 2021, based on tumor response and durability of response from the innovaTV 204 pivotal Phase 2 single-arm clinical trial evaluating TIVDAK in patients with previously treated recurrent or metastatic cervical cancer. The FDA’s approval of the sBLA converts the accelerated approval for TIVDAK to full approval in the U.S.
“Today marks a great day for patients, especially adults battling advanced cervical cancer,” said Tamika Felder, cervical cancer patient advocate, and Founder and Chief Visionary Officer, Cervivor, Inc. “This full approval opens up new treatment paths for this patient community who have long faced limited options.”
About Cervical Cancer
Cervical cancer remains a disease with high unmet need despite advances in effective vaccination and screening practices to prevent and diagnose pre-/early-stage cancers for curative treatment. Recurrent and/or metastatic cervical cancer is a particularly devastating and mostly incurable disease; up to 15% of adults with cervical cancer present with metastatic disease at diagnosisiv,v and, for adults diagnosed at earlier stages who receive treatment, up to 61%vi will experience disease recurrence. It was estimated that, in 2023, more than 13,960 new cases of invasive cervical cancer were diagnosed in the U.S. and 4,310 adults would die from the disease.vii
About the innovaTV 301 Trial
The innovaTV 301 trial (NCT04697628) is a global, 1:1 randomized, open-label Phase 3 trial evaluating TIVDAK® (tisotumab vedotin-tftv) versus investigator’s choice of single agent chemotherapy (topotecan, vinorelbine, gemcitabine, irinotecan, or pemetrexed) in 502 patients with recurrent or metastatic cervical cancer who received chemotherapy in the recurrent or metastatic setting.
Patients with recurrent or metastatic cervical cancer with squamous cell, adenocarcinoma, or adenosquamous histology, and disease progression during or after treatment with chemotherapy doublet +/- bevacizumab and an anti-PD-(L)1 agent (if eligible) are included. The primary endpoint was overall survival. The main secondary outcomes were progression-free survival and objective response rate.
The study was conducted by Seagen, which was acquired by Pfizer in December 2023, in collaboration with Genmab, European Network of Gynaecological Oncological Trial Groups (ENGOT, study number ENGOT cx-12) and the Gynecologic Oncology Group (GOG) Foundation (study number GOG 3057), as well as other global gynecological oncology cooperative groups. For more information about the Phase 3 innovaTV 301 clinical trial and other clinical trials with tisotumab vedotin, please visit .
About TIVDAK® (tisotumab vedotin-tftv)
TIVDAK® (tisotumab vedotin-tftv) is an antibody-drug conjugate (ADC) composed of Genmab’s human monoclonal antibody directed to tissue factor (TF) and Pfizer’s ADC technology that utilizes a protease-cleavable linker that covalently attaches the microtubule-disrupting agent monomethyl auristatin E (MMAE) to the antibody. Nonclinical data suggest that the anticancer activity of tisotumab vedotin-tftv is due to the binding of the ADC to TF-expressing cancer cells, followed by internalization of the ADC-TF complex, and release of MMAE via proteolytic cleavage. MMAE disrupts the microtubule network of actively dividing cells, leading to cell cycle arrest and apoptotic cell death. In vitro, tisotumab vedotin-tftv also mediates antibody-dependent cellular phagocytosis and antibody-dependent cellular cytotoxicity. TIVDAK received accelerated approval from the U.S. FDA in September 2021 for adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy.
Indication
TIVDAK is indicated for the treatment of adult patients with recurrent or metastatic cervical cancer (r/mCC) with disease progression on or after chemotherapy.
Important Safety Information
BOXED WARNING: OCULAR TOXICITY
TIVDAK can cause severe ocular toxicities resulting in changes in vision, including severe vision loss, and corneal ulceration. Conduct an ophthalmic exam, including an assessment of ocular symptoms, visual acuity, and slit lamp exam of the anterior segment of the eye prior to initiation of TIVDAK, prior to every cycle for the first nine cycles, and as clinically indicated. Adhere to the required premedication and eye care before, during, and after infusion. Withhold TIVDAK until improvement and resume, reduce the dose, or permanently discontinue, based on severity.
Warnings and Precautions
Ocular adverse reactions: TIVDAK can cause severe ocular adverse reactions, including conjunctivitis, keratopathy (keratitis, punctate keratitis, and ulcerative keratitis), and dry eye (increased lacrimation, eye pain, eye discharge, pruritus, irritation, and foreign body sensation), that may lead to changes in vision and/or corneal ulceration.
Ocular adverse reactions occurred in 55% of patients with cervical cancer treated with TIVDAK across clinical trials. The most common were conjunctivitis (32%), dry eye (24%), keratopathy (17%), and blepharitis (5%). Grade 3 ocular adverse reactions occurred in 3.3% of patients, including severe ulcerative keratitis in 1.2% of patients. Nine patients (2.1%) experienced ulcerative keratitis (including one with perforation requiring corneal transplantation), six (1.4%) conjunctival ulcer, four (0.9%) corneal erosion, two (0.5%) conjunctival erosion, and two (0.5%) symblepharon.
In innovaTV 301, 8 patients (3.2%) experienced delayed ocular adverse reactions occurring more than 30 days after discontinuation of TIVDAK. These adverse reactions included 3 patients with ulcerative keratitis, and one patient (each) with keratitis, punctate keratitis and corneal erosion, blepharitis and conjunctival hyperemia, conjunctival scar, and conjunctivitis and xerophthalmia.
Refer patients to an eye care provider to conduct an ophthalmic exam prior to initiation of TIVDAK, prior to every cycle for the first nine cycles, and as clinically indicated. The exam should include visual acuity, slit lamp exam of the anterior segment of the eye, and an assessment of normal eye movement and ocular signs or symptoms which include dry or irritated eyes, eye secretions, or blurry vision.
Adhere to the required premedication and eye care before, during, and after infusion to reduce the risk of ocular adverse reactions. Monitor for ocular toxicity and promptly refer patients to an eye care provider for any new or worsening ocular signs and symptoms. Withhold, reduce, or permanently discontinue TIVDAK based on the severity or persistence of the ocular adverse reaction.
Peripheral Neuropathy (PN) occurred in 39% of cervical cancer patients treated with TIVDAK across clinical trials; 6% of patients experienced Grade 3 PN. PN adverse reactions included peripheral sensory neuropathy (23%), PN (5%), paresthesia (3.8%), peripheral sensorimotor neuropathy (3.3%), muscular weakness (2.8%), and peripheral motor neuropathy (2.4%). One patient with another tumor type treated with TIVDAK at the recommended dose developed Guillain- Barre syndrome.
Monitor patients for signs and symptoms of neuropathy such as paresthesia, tingling or a burning sensation, neuropathic pain, muscle weakness, or dysesthesia. For new or worsening PN, withhold, then dose reduce, or permanently discontinue TIVDAK based on the severity of PN.
Hemorrhage occurred in 51% of cervical cancer patients treated with TIVDAK across clinical trials. The most common all grade hemorrhage adverse reaction was epistaxis (33%). Grade 3 hemorrhage occurred in 4% of patients.
Monitor patients for signs and symptoms of hemorrhage. For patients experiencing pulmonary or central nervous system hemorrhage, permanently discontinue TIVDAK. For Grade ≥2 hemorrhage in any other location, withhold until bleeding has resolved, blood hemoglobin is stable, there is no bleeding diathesis that could increase the risk of continuing therapy, and there is no anatomical or pathologic condition that can increase the risk of hemorrhage recurrence. After resolution, either resume treatment or permanently discontinue TIVDAK.
Pneumonitis that is severe, life-threatening, or fatal can occur in patients treated with antibody-drug conjugates containing vedotin, including TIVDAK. Among cervical cancer patients treated with TIVDAK across clinical trials, 4 patients (0.9%) experienced pneumonitis, including 1 patient who had a fatal outcome.
Monitor patients for pulmonary symptoms of pneumonitis. Symptoms may include hypoxia, cough, dyspnea or interstitial infiltrates on radiologic exams. Infectious, neoplastic, and other causes for such symptoms should be excluded through appropriate investigations. Withhold TIVDAK for patients who develop persistent or recurrent Grade 2 pneumonitis and consider dose reduction. Permanently discontinue TIVDAK in all patients with Grade 3 or 4 pneumonitis.
Severe cutaneous adverse reactions (SCAR), including events of fatal or life-threatening Stevens-Johnson syndrome (SJS), can occur in patients treated with TIVDAK. SCAR occurred in 1.6% of cervical cancer patients treated with TIVDAK across clinical trials. Grade ≥3 SCAR occurred in 0.5% of patients, including 1 patient who had a fatal outcome.
Monitor patients for signs or symptoms of SCAR, which include target lesions, worsening skin reactions, blistering or peeling of the skin, painful sores in mouth, nose, throat, or genital area, fever or flu-like symptoms, and swollen lymph nodes. If signs or symptoms of SCAR occur, withhold TIVDAK until the etiology of the reaction has been determined. Early consultation with a specialist is recommended to ensure greater diagnostic accuracy and appropriate management. Permanently discontinue TIVDAK for confirmed Grade 3 or 4 SCAR, including SJS.
Embryo-fetal toxicity: TIVDAK can cause fetal harm when administered to a pregnant woman. Advise patients of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TIVDAK and for 2 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TIVDAK and for 4 months after the last dose.
Adverse Reactions
Across clinical trials of TIVDAK in 425 patients with r/mCC, the most common (≥25%) adverse reactions, including laboratory abnormalities, were hemoglobin decreased (45%), PN (39%), conjunctival adverse reactions (38%), nausea (37%), fatigue (36%), aspartate aminotransferase increased (33%), epistaxis (33%), alopecia (31%), alanine aminotransferase increased (30%), and hemorrhage (28%).
innovaTV 301 Study: 250 patients with r/mCC with disease progression on or after systemic therapy
Serious adverse reactions occurred in 33% of patients receiving TIVDAK; the most common (≥2%) were urinary tract infection (4.8%), small intestinal obstruction (2.4%), sepsis, abdominal pain, and hemorrhage (each 2%). Fatal adverse reactions occurred in 1.6% of patients who received TIVDAK, including acute kidney injury, pneumonia, sepsis, and SJS (each 0.4%).
Adverse reactions leading to permanent discontinuation occurred in 15% of patients receiving TIVDAK; the most common (≥3%) were PN and ocular adverse reactions (each 6%). Adverse reactions leading to dose interruption occurred in 39% of patients receiving TIVDAK; the most common (≥3%) were ocular adverse reactions (16%) and PN (6%). Adverse reactions leading to dose reduction occurred in 30% of patients receiving TIVDAK; the most common (≥3%) were PN and ocular adverse reactions (each 10%). The ocular adverse reactions included conjunctival disorders (4.8%), keratopathy (4%), and dry eye (0.8%).
innovaTV 204 Study: 101 patients with r/mCC with disease progression on or after chemotherapy
Serious adverse reactions occurred in 43% of patients; the most common (≥3%) were ileus (6%), hemorrhage (5%), pneumonia (4%), PN, sepsis, constipation, and pyrexia (each 3%). Fatal adverse reactions occurred in 4% of patients who received TIVDAK, including septic shock, pneumonitis, sudden death, and multisystem organ failure (each 1%).
Adverse reactions leading to permanent discontinuation occurred in 13% of patients receiving TIVDAK; the most common (≥3%) were PN (5%) and corneal adverse reactions (4%). Adverse reactions leading to dose interruption occurred in 47% of patients; the most common (≥3%) were PN (8%), conjunctival adverse reactions, and hemorrhage (each 4%). Adverse reactions leading to dose reduction occurred in 23% of patients; the most common (≥3%) were conjunctival adverse reactions (9%) and corneal adverse reactions (8%).
Drug Interactions
Strong CYP3A4 inhibitors: Concomitant use with strong CYP3A4 inhibitors may increase unconjugated monomethyl auristatin E (MMAE) exposure, which may increase the risk of TIVDAK adverse reactions. Closely monitor patients for TIVDAK adverse reactions.
Use in Specific Populations
Moderate or severe hepatic impairment: MMAE exposure and adverse reactions are increased. Avoid use.
Lactation: Advise lactating women not to breastfeed during TIVDAK treatment and for at least 3 weeks after the last dose.
Please see full prescribing information, including BOXED WARNING for TIVDAK here.
About Pfizer Oncology
At Pfizer Oncology, we are at the forefront of a new era in cancer care. Our industry-leading portfolio and extensive pipeline includes game-changing mechanisms of action to attack cancer from multiple angles, including antibody-drug conjugates (ADCs), small molecules, bispecific antibodies and other immunotherapy biologics. We are focused on delivering transformative therapies in some of the world’s most common cancers, including breast cancer, genitourinary cancer, hematology-oncology and thoracic cancers, which includes lung cancer. Driven by science, we are committed to accelerating breakthroughs to extend and improve patients’ lives.
About Genmab
Genmab is an international biotechnology company with a core purpose guiding its unstoppable team to strive towards improving the lives of patients through innovative and differentiated antibody therapeutics. For 25 years, its passionate, innovative and collaborative team has invented next-generation antibody technology platforms and leveraged translational, quantitative, and data sciences, resulting in a proprietary pipeline including bispecific T-cell engagers, next-generation immune checkpoint modulators, effector function enhanced antibodies, and antibody-drug conjugates. To help develop and deliver novel antibody therapies to patients, Genmab has formed 20+ strategic partnerships with biotechnology and pharmaceutical companies. By 2030, Genmab’s vision is to transform the lives of people with cancer and other serious diseases with knock-your-socks-off (KYSO®) antibody medicines.
Established in 1999, Genmab is headquartered in Copenhagen, Denmark with locations in Utrecht, the Netherlands, Princeton, New Jersey, U.S., and Tokyo, Japan. For more information, please visit Genmab.com and follow us on LinkedIn and X.
About the Pfizer and Genmab Collaboration
Tisotumab vedotin is co-owned by Genmab and Pfizer, under an agreement in which the companies share costs and profits for the product on a 50:50 basis.
Pfizer Disclosure Notice
The information contained in this release is as of April 29, 2024. Pfizer assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.
This release contains forward-looking information about Pfizer Oncology and TIVDAK® (tisotumab vedotin-tftv), including its potential benefits and its ongoing clinical development program, that involves substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. Risks and uncertainties include, among other things, uncertainties regarding the commercial success of TIVDAK; the uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for our clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates, as well as the possibility of unfavorable new clinical data and further analyses of existing clinical data; the risk that clinical trial data are subject to differing interpretations and assessments by regulatory authorities; whether regulatory authorities will be satisfied with the design of and results from our clinical studies; whether and when drug applications may be filed in particular jurisdictions for TIVDAK; whether and when any applications that may be pending or filed for TIVDAK may be approved by regulatory authorities, which will depend on myriad factors, including making a determination as to whether the product's benefits outweigh its known risks and determination of the product's efficacy and, if approved, whether TIVDAK will be commercially successful; decisions by regulatory authorities impacting labeling, manufacturing processes, safety and/or other matters that could affect the availability or commercial potential of TIVDAK; whether the collaboration between Pfizer and Genmab will be successful; uncertainties regarding the impact of COVID-19 on Pfizer’s business, operations and financial results; and competitive developments.
A further description of risks and uncertainties can be found in Pfizer’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023 and in its subsequent reports on Form 10-Q, including in the sections thereof captioned “Risk Factors” and “Forward-Looking Information and Factors That May Affect Future Results”, as well as in its subsequent reports on Form 8-K, all of which are filed with the U.S. Securities and Exchange Commission and available at and .
Genmab Forward Looking Statements
This Company Announcement contains forward looking statements. The words “believe”, “expect”, “anticipate”, “intend” and “plan” and similar expressions identify forward looking statements. Actual results or performance may differ materially from any future results or performance expressed or implied by such statements. The important factors that could cause our actual results or performance to differ materially include, among others, risks associated with pre-clinical and clinical development of products, uncertainties related to the outcome and conduct of clinical trials including unforeseen safety issues, uncertainties related to product manufacturing, the lack of market acceptance of our products, our inability to manage growth, the competitive environment in relation to our business area and markets, our inability to attract and retain suitably qualified personnel, the unenforceability or lack of protection of our patents and proprietary rights, our relationships with affiliated entities, changes and developments in technology which may render our products or technologies obsolete, and other factors. For a further discussion of these risks, please refer to the risk management sections in Genmab’s most recent financial reports, which are available on the risk factors included in Genmab’s most recent Annual Report on Form 20-F and other filings with the U.S. Securities and Exchange Commission (SEC), which are available at . Genmab does not undertake any obligation to update or revise forward looking statements in this Company Announcement nor to confirm such statements to reflect subsequent events or circumstances after the date made or in relation to actual results, unless required by law.
Genmab A/S and/or its subsidiaries own the following trademarks: Genmab®; the Y-shaped Genmab logo®; Genmab in combination with the Y-shaped Genmab logo®; HuMax®; DuoBody®; HexaBody®; DuoHexaBody® and HexElect®. TIVDAK® is a trademark of Pfizer Inc.
i The threshold for statistical significance is 0.0226 (two-sided).
ii Cervical Cancer: Statistics. American Society of Clinical Oncology (ASCO). September 2023.
iii Priority Review. U.S. Food and Drug Administration. January 4, 2018.
iv National Cancer Institute. SEER Cancer Stat Facts: Cervical Cancer. 2023.
v McLachlan J, Boussios S, Okines A, et al. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin Oncol (R Coll Radiol). 2017;29(3):153-60.
vi Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016 Jan;214(1):22-30. doi: 10.1016/j.ajog.2015.07.022. Epub 2015 Jul 26. PMID: 26212178; PMCID: PMC5613936.
vii Key Statistics for Cervical Cancer. American Cancer Society. Atlanta, GA. 2023.
Phase 3Clinical ResultDrug ApprovalAccelerated ApprovalPhase 2
100 Deals associated with Topotecan Hydrochloride
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D02168 | Topotecan Hydrochloride |
R&D Status
Approved
10 top approved records. to view more data
Login
| Indication | Country/Location | Organization | Date |
|---|---|---|---|
| Solid tumor | JP | 20 Nov 2013 | |
| Ovarian Epithelial Carcinoma | LI | 06 Jan 2011 | |
| Ovarian Epithelial Carcinoma | IS | 06 Jan 2011 | |
| Ovarian Epithelial Carcinoma | NO | 06 Jan 2011 | |
| Ovarian Epithelial Carcinoma | EU | 06 Jan 2011 | |
| Non-Small Cell Lung Cancer | JP | 06 Mar 2003 | |
| Small cell lung cancer recurrent | NO | - | 12 Nov 1996 |
| Small cell lung cancer recurrent | EU | - | 12 Nov 1996 |
| Small cell lung cancer recurrent | IS | - | 12 Nov 1996 |
| Small cell lung cancer recurrent | LI | - | 12 Nov 1996 |
| Ovarian Cancer | US | 28 May 1996 | |
| Small Cell Lung Cancer | US | 28 May 1996 | |
| Uterine Cervical Cancer | US | 28 May 1996 |
Developing
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Small Cell Lung Cancer | Phase 3 | NO | 21 Sep 2009 | |
| Small Cell Lung Cancer | Phase 3 | EU | 21 Sep 2009 | |
| Small Cell Lung Cancer | Phase 3 | LI | 21 Sep 2009 | |
| Small Cell Lung Cancer | Phase 2 | LI | 21 Sep 2009 | |
| Small Cell Lung Cancer | Phase 2 | NO | 21 Sep 2009 | |
| Small Cell Lung Cancer | Phase 1 | EU | 21 Sep 2009 | |
| Small Cell Lung Cancer | Discovery | IS | 21 Sep 2009 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 6 | (solid tumours) | (yixuogpmmu) = pcibgivmfh mvgpdirqzn (bzemwkaybu ) View more | Positive | 10 Sep 2022 | ||
( pt-resistant SCLC) | (yixuogpmmu) = bkxqhfylwp mvgpdirqzn (bzemwkaybu ) View more | ||||||
Phase 3 | 13 | (ddrwhykvxa) = jbasnmphki ijyxciboaz (mtsvbiclmx ) View more | Positive | 01 Dec 2022 | |||
(first-relapse Ewing sarcoma patients) | (ddrwhykvxa) = hsbxhydvwz ijyxciboaz (mtsvbiclmx ) View more | ||||||
AcSé-ESMART (Pubmed) Manual | Phase 1/2 | 32 | (yeudocdncz) = The recommended phase II dose was ribociclib 260 mg/m2 once a day, temozolomide 100 mg/m2 once a day, and topotecan 0.5 mg/m2 once a day (arm A) and ribociclib 175 mg/m2 once a day and everolimus 2.5 mg/m2 once a day (arm B). mqeutrccqh (jukksoknua ) View more | Positive | 10 Nov 2021 | ||
Phase 1 | 44 | (jucfswtfvc) = ijscgohcph dyklvybigx (wjxvltpucp ) View more | Positive | 16 Sep 2021 | |||
(jucfswtfvc) = hzqrtspuhp dyklvybigx (wjxvltpucp ) View more | |||||||
NCT02303028 (Pubmed) Manual | Phase 1 | 30 | (gtwxecdeuu) = 0.22 mg/m2 topotecan and 160 mg/m2 pazopanib qdcxozuedc (luyiskxwvk ) View more | Negative | 17 Jun 2022 | ||
NCT03794349 (Literature) Manual | Phase 1 | 24 | (korcizohni) = puzdrekwcx ecoujnixzq (hvdwffpmso ) View more | Positive | 10 Jan 2024 | ||
Phase 2 | Recurrent ovarian cancer Third line | 70 | (ktxmhkunry) = ouukvbrpzu cdwpdaluuj (jqxcbxsbma, 24.7 - 75.4) View more | Positive | 20 May 2021 | ||
(O+D) | (ktxmhkunry) = jodcidcrzc cdwpdaluuj (jqxcbxsbma, 12.8 - 64.9) View more | ||||||
Phase 1 | 14 | (ygfumamror) = xindowizvr iisfcorodp (bcipafdqnf ) View more | Positive | 01 Oct 2021 | |||
NCT04047251 (ASCO2022) Manual | Phase 1 | 29 | (lddjevmvfu) = uurmqsnolj vnaduxrnlr (rjwiealdve ) View more | Positive | 02 Jun 2022 | ||
Phase 3 | 451 | (klwtrzvyhy) = rjqssqweam qkgvhyemti (ogtrncaskb, 2.1 - 6.2) View more | Positive | 08 Jun 2022 | |||
irinotecan + temolozomide | - |
Login to view more data
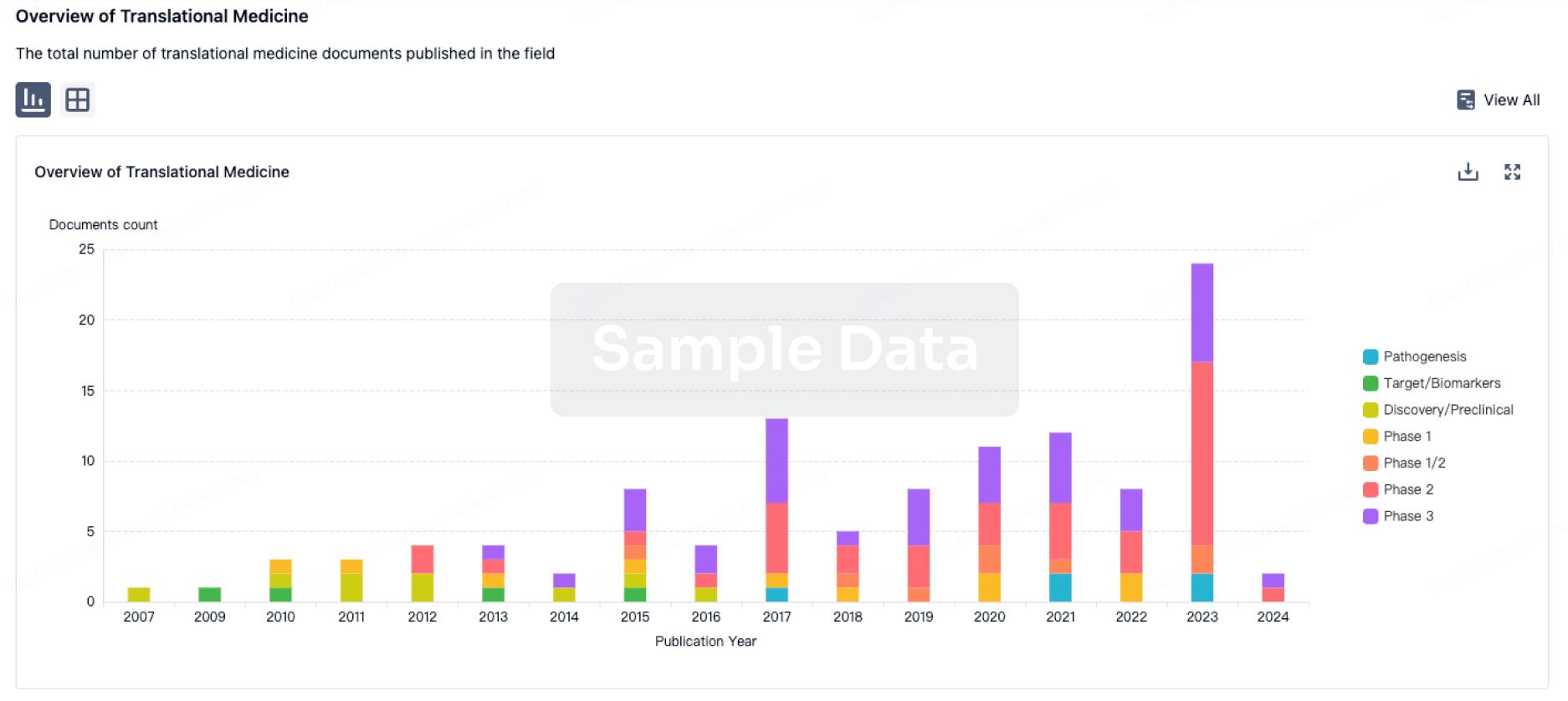
Translational Medicine
Boost your research with our translational medicine data.
login
or
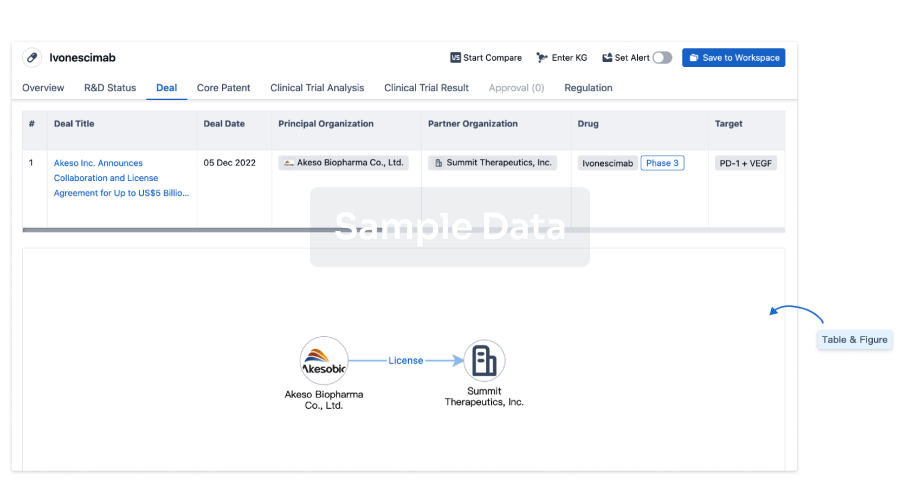
Deal
Boost your decision using our deal data.
login
or
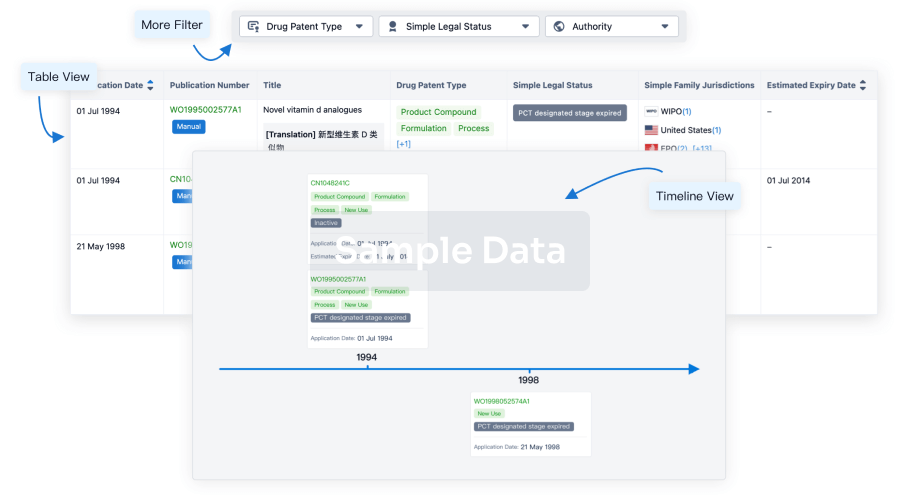
Core Patent
Boost your research with our Core Patent data.
login
or
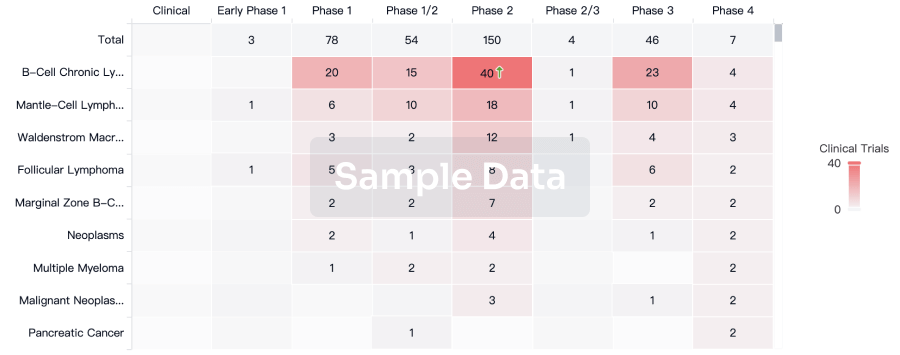
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free






