KIN-2787: A Next-Generation Pan-RAF Inhibitor Effective Against Class II and III BRAF Mutant Cancers
KIN-2787 is a novel, oral, and selective pan-RAF inhibitor developed to target both Class I and the less responsive Class II and III BRAF dimers. The drug's efficacy and safety were tested in various BRAF mutation-driven cancer models both in the laboratory and in live organisms.
In laboratory assays, KIN-2787 demonstrated high potency against RAF1, BRAF, and ARAF proteins with IC50 values ranging from 0.06 to 3.46 nanomolar, showing minimal interaction with non-RAF kinases. It effectively inhibited RAF activity and downstream ERK phosphorylation in multiple BRAF mutant cancer cell lines, with Class II and III BRAF mutants showing the highest sensitivity to the treatment.
In animal models, KIN-2787 displayed a dose-dependent inhibition of tumor growth in BRAF mutant human xenograft models, with daily dosing being well-tolerated. Twice daily dosing showed a trend toward greater tumor response compared to once daily dosing, but both dosing schedules resulted in significant tumor growth inhibition and regression at equivalent total daily doses.
The drug also induced a significant pharmacodynamic response in vivo, with twice daily dosing achieving more sustained target engagement. Additional effects of KIN-2787 on biomarkers and modulation of the MAPK pathway will be discussed in an upcoming meeting.
In conclusion, KIN-2787 is a promising next-generation pan-RAF inhibitor with notable activity against cancers with Class II and III BRAF mutations. A phase 1 clinical trial to assess the safety and efficacy of KIN-2787 as a monotherapy for patients with advanced solid tumors, including those with Class II and III BRAF mutations, is anticipated to start in 2021.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
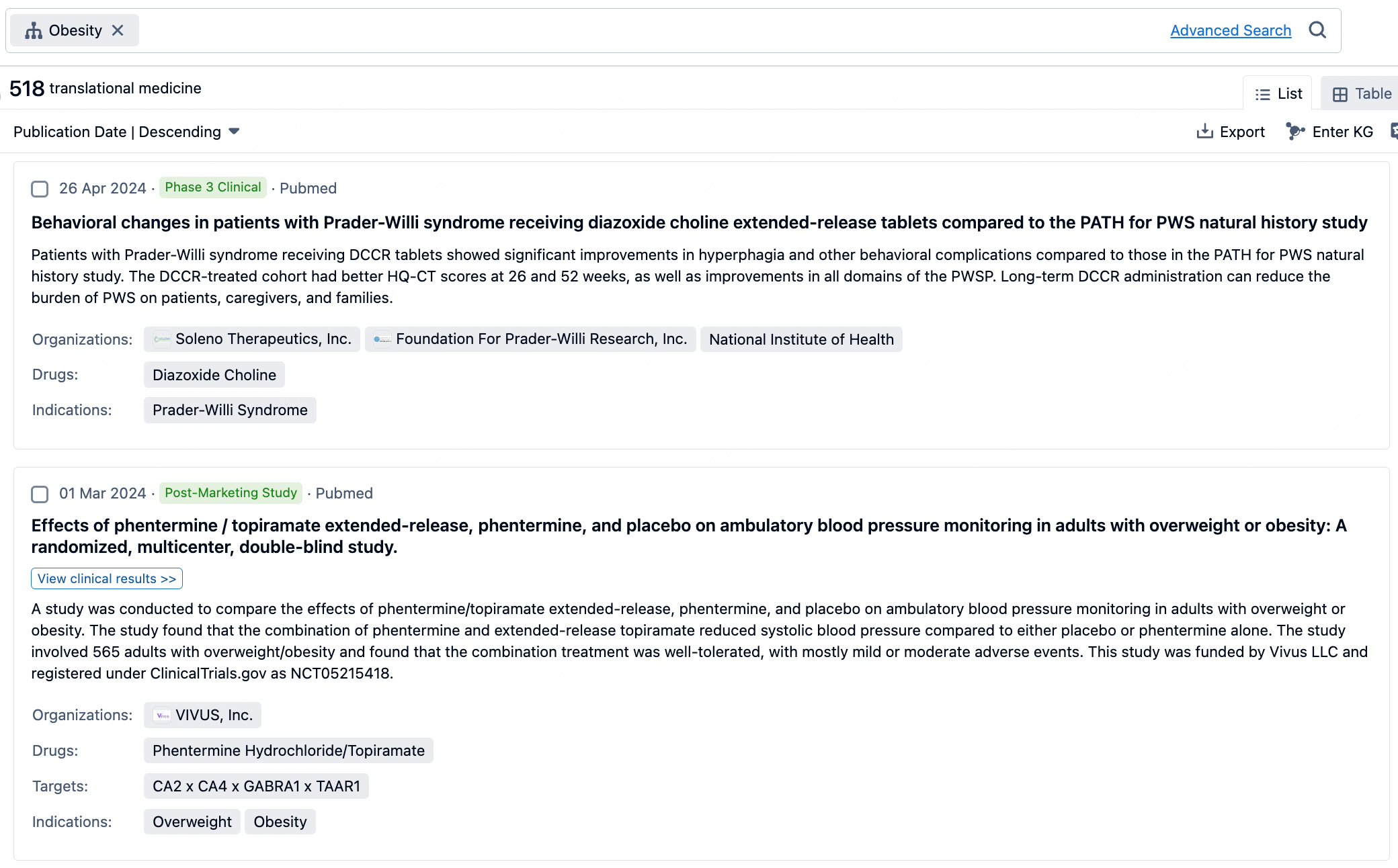
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
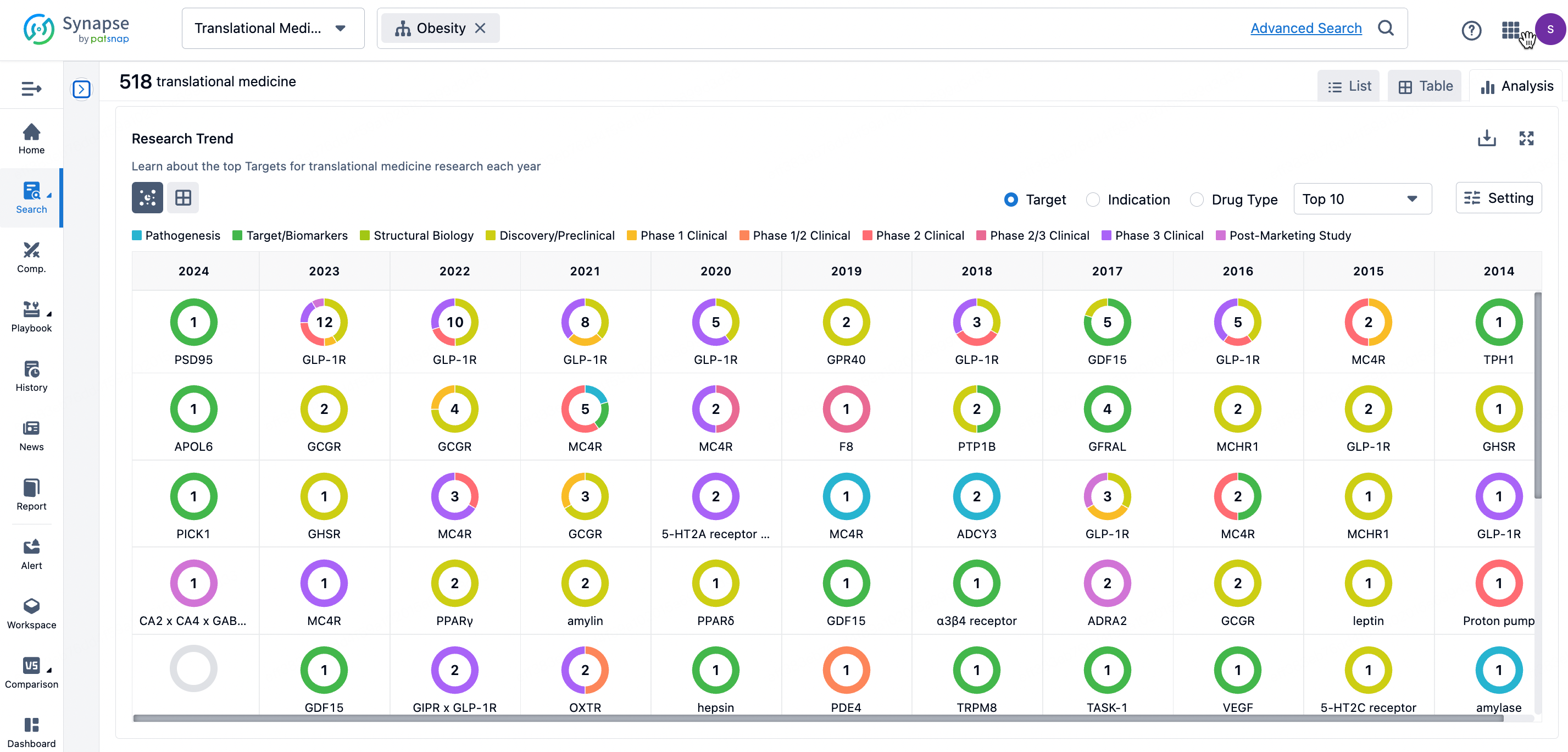
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.