Request Demo
What are PI3Kδ inhibitors and how do they work?
21 June 2024
The PI3Kδ (phosphoinositide 3-kinase delta) inhibitors represent a significant advancement in the field of targeted cancer therapies. These drugs specifically inhibit a subtype of the PI3K enzyme, which plays a critical role in multiple cellular functions, including cell growth, proliferation, differentiation, motility, and survival. PI3Kδ, in particular, is predominantly expressed in leukocytes, which makes it a compelling target for treating hematological malignancies and other immune-related disorders.
PI3Kδ inhibitors work by blocking the activity of the PI3Kδ enzyme. PI3K enzymes are part of the larger PI3K/AKT/mTOR signaling pathway, which is crucial for regulating cellular growth and survival. When PI3Kδ is inhibited, the downstream signaling is disrupted, leading to reduced cell proliferation and increased apoptosis (programmed cell death). This specific inhibition is particularly beneficial in targeting B-cell malignancies, as B-cells rely heavily on PI3Kδ signaling for their growth and survival.
The inhibition process involves binding the PI3Kδ inhibitors to the ATP-binding pocket of the PI3Kδ enzyme, thereby preventing its activation. Consequently, the downstream signaling that promotes cell survival and proliferation is halted. This mechanism of action not only curbs tumor growth but also enhances the immune response against cancer cells. Furthermore, by selectively targeting PI3Kδ rather than other PI3K isoforms, these inhibitors minimize the adverse effects on normal cells, thus offering a more targeted and less toxic therapeutic option.
PI3Kδ inhibitors are primarily used for treating certain types of blood cancers, including chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and follicular lymphoma (FL). These cancers are characterized by the malignant growth of B-cells, which are a type of white blood cell integral to the immune system. The overactivation of the PI3K/AKT/mTOR pathway is a common feature in these malignancies, making PI3Kδ a strategic target for therapy.
One of the first PI3Kδ inhibitors to be approved by the FDA is idelalisib (Zydelig). Idelalisib has demonstrated significant efficacy in patients with relapsed or refractory CLL and FL. Clinical trials have shown that idelalisib, when combined with other treatments such as rituximab, improves progression-free survival and overall response rates in patients. Other PI3Kδ inhibitors, such as duvelisib (Copiktra) and umbralisib, have also shown promise in clinical settings, offering new hope for patients who have not responded to conventional therapies.
Beyond hematological malignancies, PI3Kδ inhibitors are being explored for their potential in treating autoimmune diseases. Since PI3Kδ is predominantly expressed in leukocytes, its inhibition can modulate immune responses, making it a potential therapeutic target for conditions like rheumatoid arthritis and systemic lupus erythematosus. Early-stage clinical trials are underway to evaluate the efficacy and safety of PI3Kδ inhibitors in these autoimmune conditions.
In conclusion, PI3Kδ inhibitors represent a powerful class of targeted therapies with the potential to revolutionize the treatment of certain hematological malignancies and autoimmune diseases. By specifically targeting the PI3Kδ isoform, these inhibitors provide a more precise approach to cancer treatment, reducing adverse effects and improving patient outcomes. As research continues to advance, the therapeutic applications of PI3Kδ inhibitors are likely to expand, offering new hope for patients with various challenging conditions.
PI3Kδ inhibitors work by blocking the activity of the PI3Kδ enzyme. PI3K enzymes are part of the larger PI3K/AKT/mTOR signaling pathway, which is crucial for regulating cellular growth and survival. When PI3Kδ is inhibited, the downstream signaling is disrupted, leading to reduced cell proliferation and increased apoptosis (programmed cell death). This specific inhibition is particularly beneficial in targeting B-cell malignancies, as B-cells rely heavily on PI3Kδ signaling for their growth and survival.
The inhibition process involves binding the PI3Kδ inhibitors to the ATP-binding pocket of the PI3Kδ enzyme, thereby preventing its activation. Consequently, the downstream signaling that promotes cell survival and proliferation is halted. This mechanism of action not only curbs tumor growth but also enhances the immune response against cancer cells. Furthermore, by selectively targeting PI3Kδ rather than other PI3K isoforms, these inhibitors minimize the adverse effects on normal cells, thus offering a more targeted and less toxic therapeutic option.
PI3Kδ inhibitors are primarily used for treating certain types of blood cancers, including chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and follicular lymphoma (FL). These cancers are characterized by the malignant growth of B-cells, which are a type of white blood cell integral to the immune system. The overactivation of the PI3K/AKT/mTOR pathway is a common feature in these malignancies, making PI3Kδ a strategic target for therapy.
One of the first PI3Kδ inhibitors to be approved by the FDA is idelalisib (Zydelig). Idelalisib has demonstrated significant efficacy in patients with relapsed or refractory CLL and FL. Clinical trials have shown that idelalisib, when combined with other treatments such as rituximab, improves progression-free survival and overall response rates in patients. Other PI3Kδ inhibitors, such as duvelisib (Copiktra) and umbralisib, have also shown promise in clinical settings, offering new hope for patients who have not responded to conventional therapies.
Beyond hematological malignancies, PI3Kδ inhibitors are being explored for their potential in treating autoimmune diseases. Since PI3Kδ is predominantly expressed in leukocytes, its inhibition can modulate immune responses, making it a potential therapeutic target for conditions like rheumatoid arthritis and systemic lupus erythematosus. Early-stage clinical trials are underway to evaluate the efficacy and safety of PI3Kδ inhibitors in these autoimmune conditions.
In conclusion, PI3Kδ inhibitors represent a powerful class of targeted therapies with the potential to revolutionize the treatment of certain hematological malignancies and autoimmune diseases. By specifically targeting the PI3Kδ isoform, these inhibitors provide a more precise approach to cancer treatment, reducing adverse effects and improving patient outcomes. As research continues to advance, the therapeutic applications of PI3Kδ inhibitors are likely to expand, offering new hope for patients with various challenging conditions.
How to obtain the latest development progress of all targets?
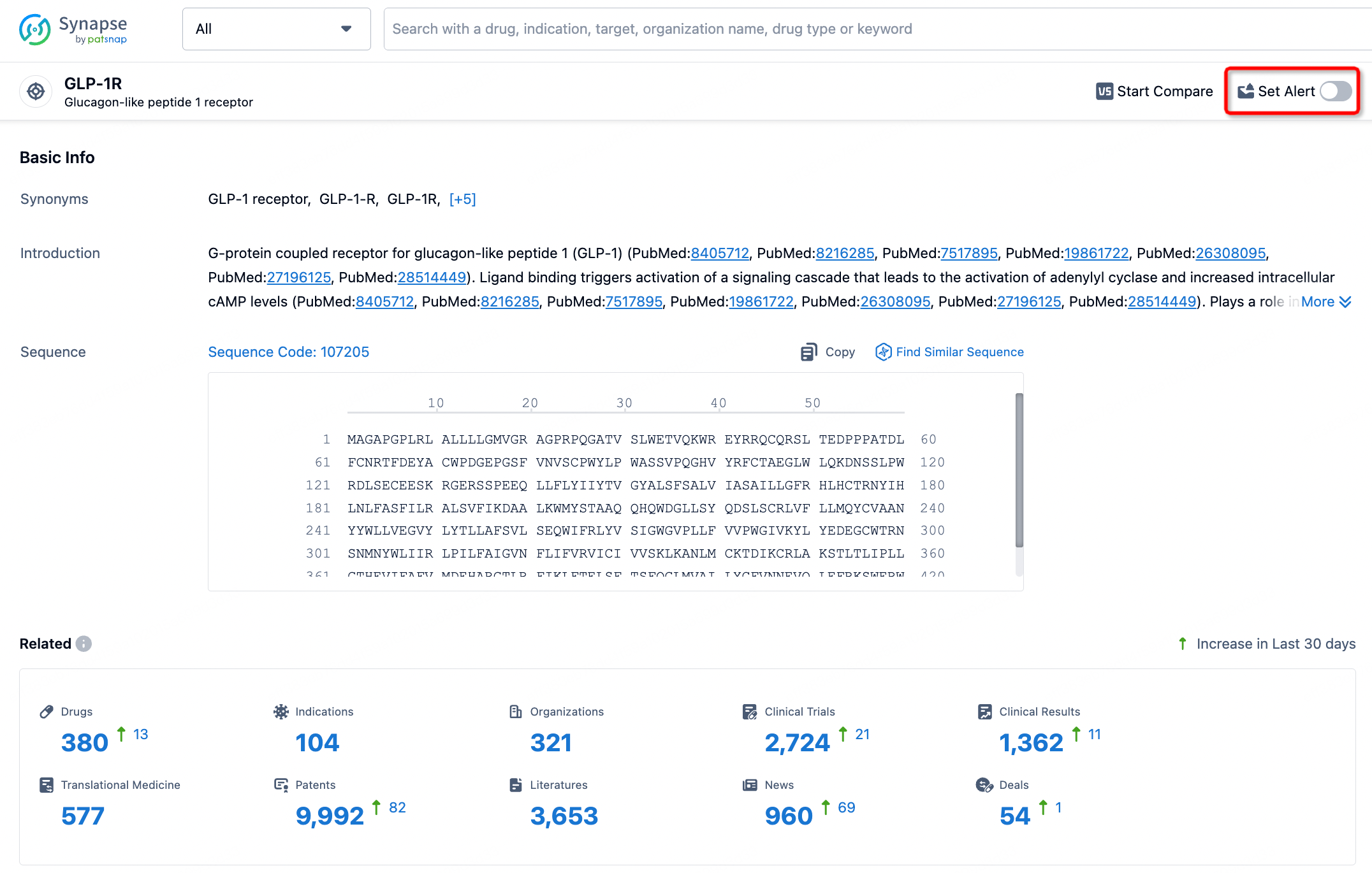
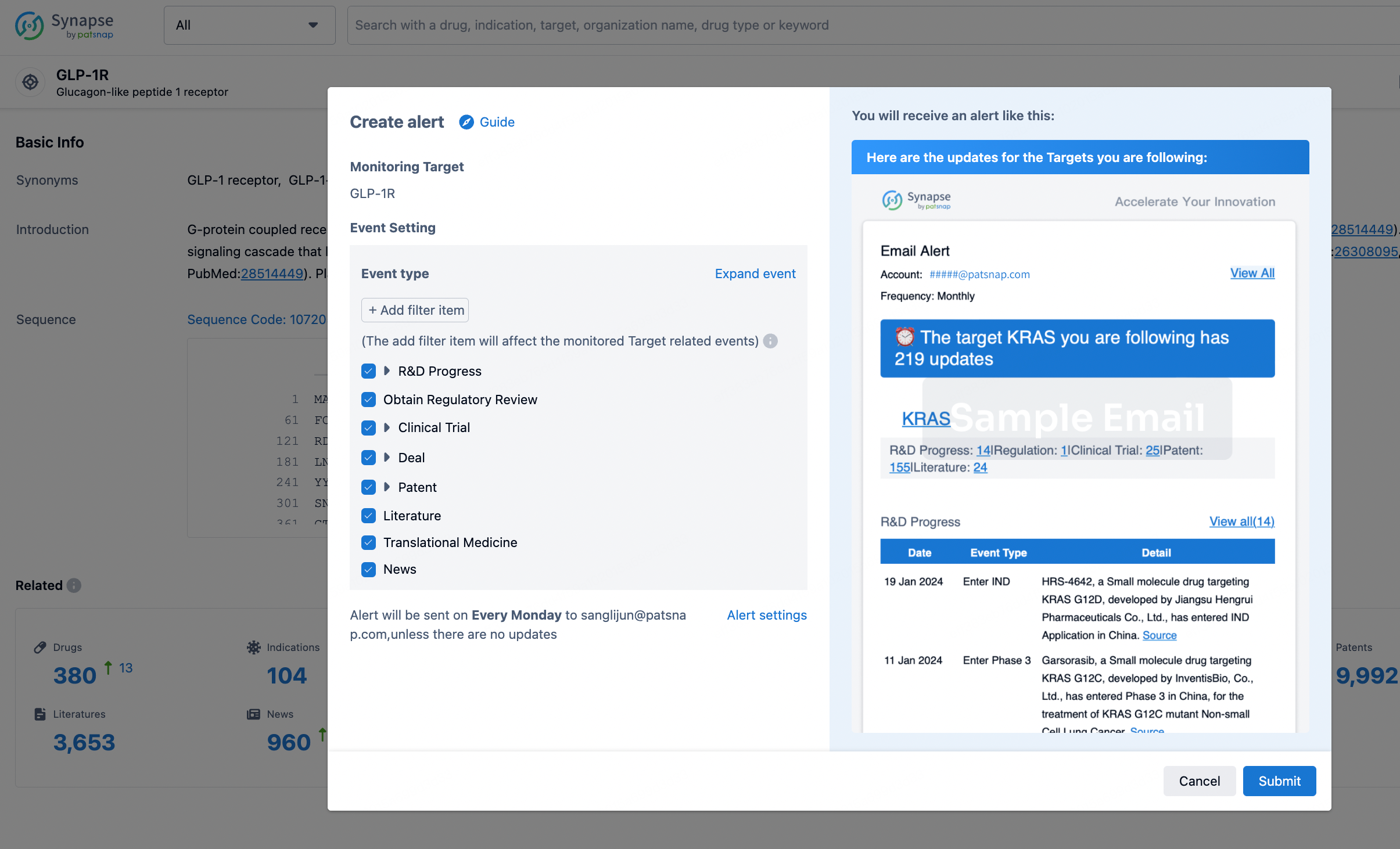
In the Synapse database, you can stay updated on the latest research and development advances of all targets. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.