[177Lu]Lu-PSMA-617: Brief Review of its R&D progress and the clinical outcome in 2023 ESMO
[177Lu]Lu-PSMA-617 (177Lu-PSMA-617) prolongs rPFS and OS in patients (pts) with mCRPC and prior ARPI and taxane therapy, but the efficacy was unknown in patients not treated with taxane. And the answers were provided at this ESCO Congress.
[177Lu]Lu-PSMA-617's R&D Progress
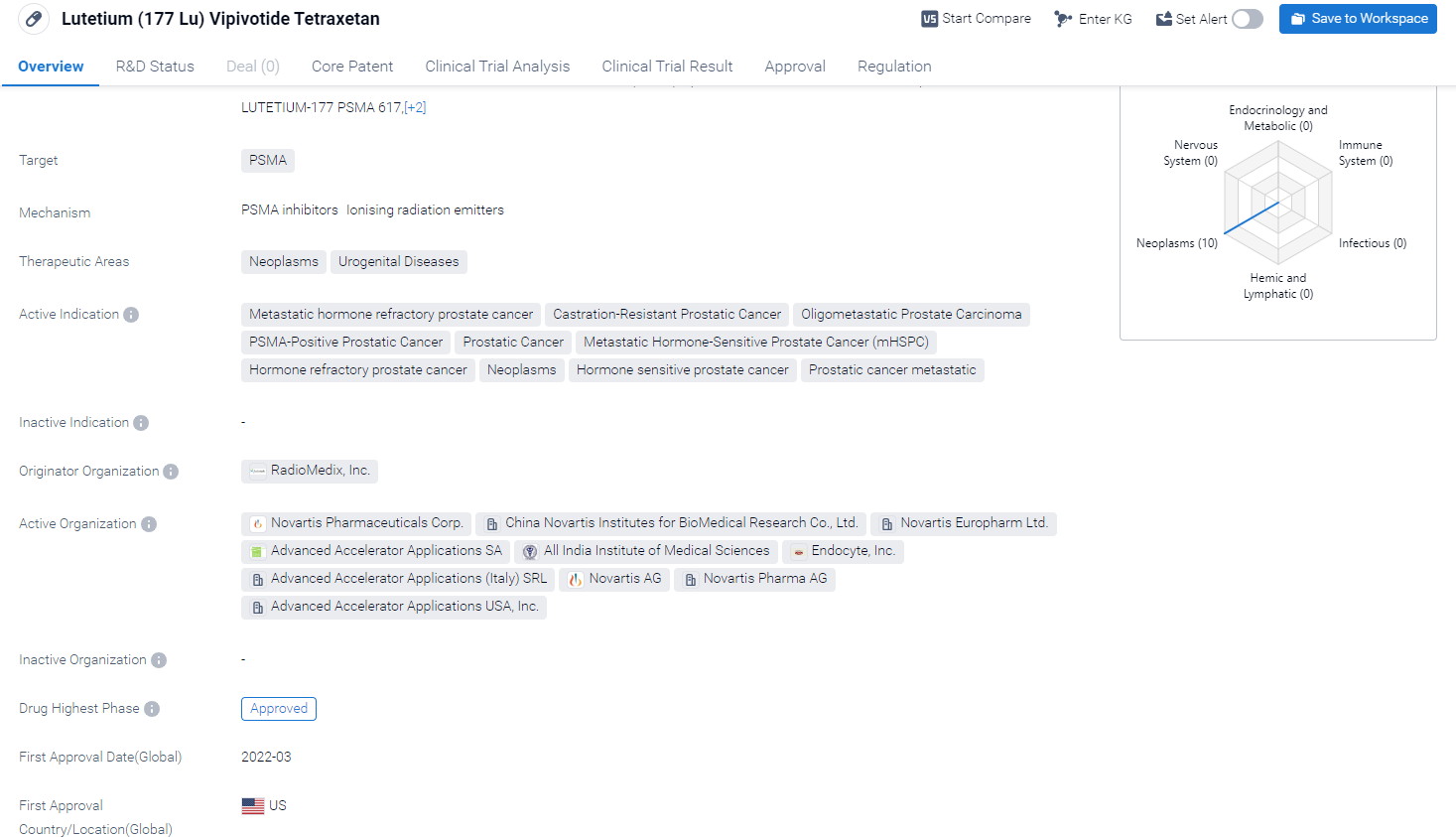
[177Lu]Lu-PSMA-617 is a drug that falls under the category of peptide drug conjugates, radionuclide drug conjugates (RDC), and therapeutic radiopharmaceuticals. It is designed to target PSMA (Prostate-Specific Membrane Antigen), making it particularly relevant in the treatment of neoplasms and urogenital diseases.
The drug has shown promising results in the treatment of various prostate cancer indications. These include metastatic hormone refractory prostate cancer, castration-resistant prostatic cancer, oligometastatic prostate carcinoma, PSMA-positive prostatic cancer, prostatic cancer, metastatic hormone-sensitive prostate cancer (mHSPC), hormone refractory prostate cancer, hormone-sensitive prostate cancer, and prostatic cancer metastatic.
According to the Patsnap Synapse, [177Lu]Lu-PSMA-617 has reached the highest phase of development, with global approval. In China, it is currently in Phase 3 of clinical trials. And the clinical trial distributions for [177Lu]Lu-PSMA-617 are primarily in the United States, China, and United Kingdom. The key indication is Metastatic hormone refractory prostate cancer.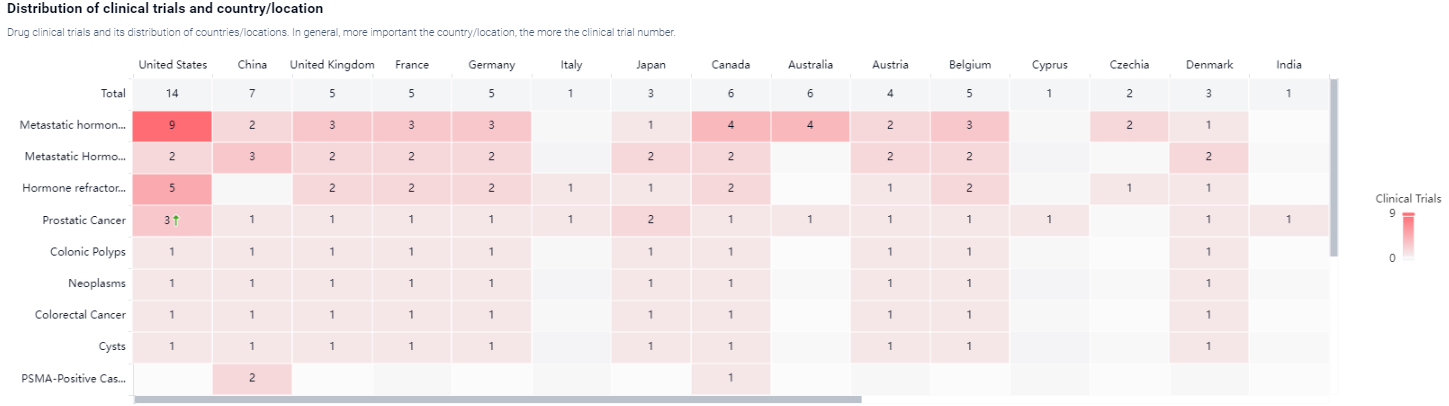
Detailed Clinical Outcome of [177Lu]Lu-PSMA-617
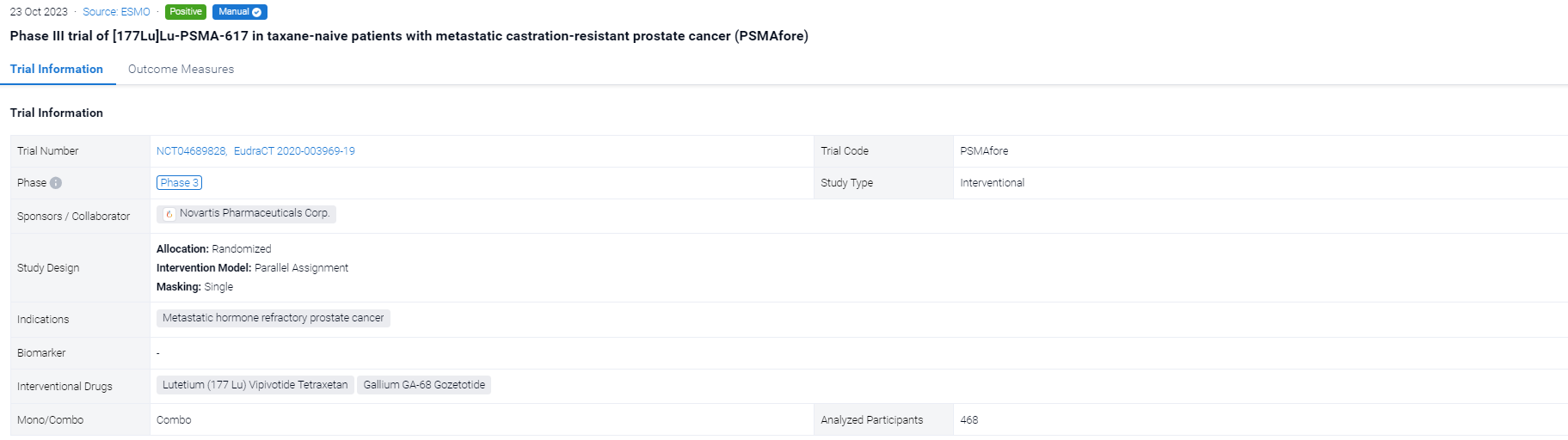
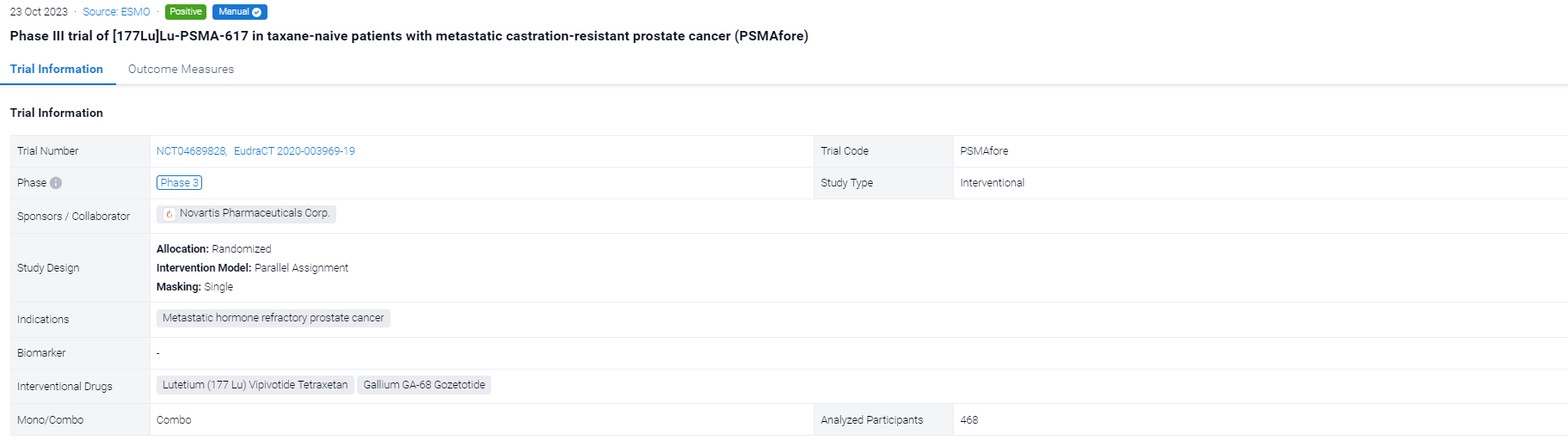
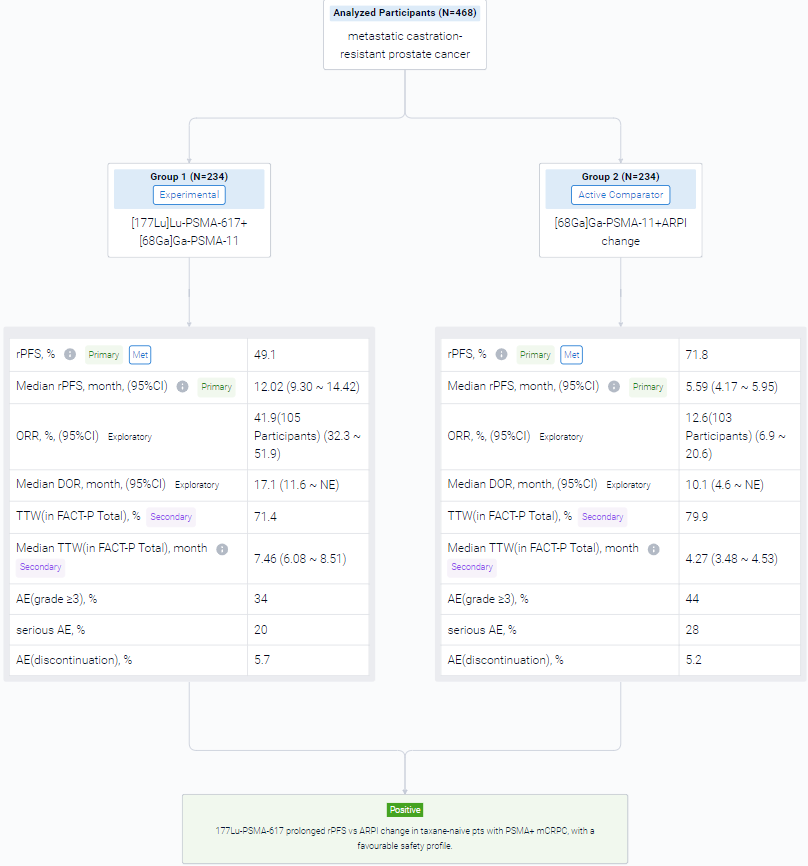
The randomized, parallel assignment, single clinical trial (NCT04689828) was aimed to examined the efficacy of 177Lu-PSMA-617 in taxane-naive pts.
In this study, eligible adults had mCRPC, were candidates for ARPI change after one progression on prior ARPI, and had ≥1 PSMA+ and no exclusionary PSMA– lesions by [68Ga]Ga-PSMA-11 PET/CT. Candidates for PARP inhibition and pts with prior systemic radiotherapy (<6 months ago), immunotherapy (except sipuleucel-T), or chemotherapy (except [neo]adjuvant >12 months ago) were ineligible. Randomization was 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq q6w; 6 cycles) or ARPI change (abiraterone/enzalutamide). Pts randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression (rPD). Endpoints included: rPFS (PCWG3/RECIST v1.1; primary), OS (key secondary) (both overall α=0.025, one-sided), FACT-P (secondary) and ORR/DOR (exploratory). Primary analysis was to occur at ∼156 rPFS events and second OS interim analysis (IA) at ~125 deaths. Crossover-adjusted analysis was the prespecified method for OS by rank-preserving structural failure time (RPSFT).
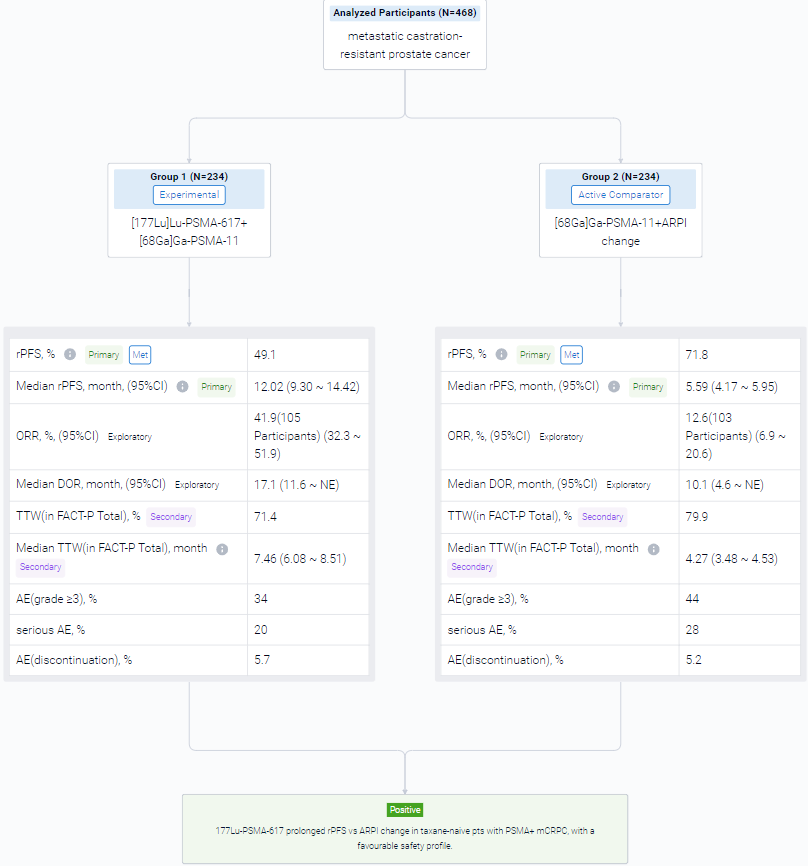
The result showed that at primary analysis (median follow-up, 7.3 months; N = 467), the primary endpoint of rPFS was met (HR, 0.41; 95% CI: 0.29, 0.56; p<0.0001); this was similar at second IA (table). At second IA (45.1% of target deaths), 123/146 (84.2%) pts with rPD who discontinued ARPI crossed over; there was a positive OS trend in favour of 177Lu-PSMA-617 per RPFST but not per unadjusted OS analysis. FACT-P and ORR/DOR favoured the 177Lu-PSMA-617 arm (table). For 177Lu-PSMA-617 vs ARPI change, incidence of grade ≥3 AEs was 34% (most common: anaemia, dry mouth) vs 44%, serious AEs 20% vs 28%, and AEs leading to discontinuation 5.7% vs 5.2%.
It can be concluded that 177Lu-PSMA-617 prolonged rPFS vs ARPI change in taxane-naive pts with PSMA+ mCRPC, with a favourable safety profile.
How to Easily View the Clinical Results Using Synapse Database?
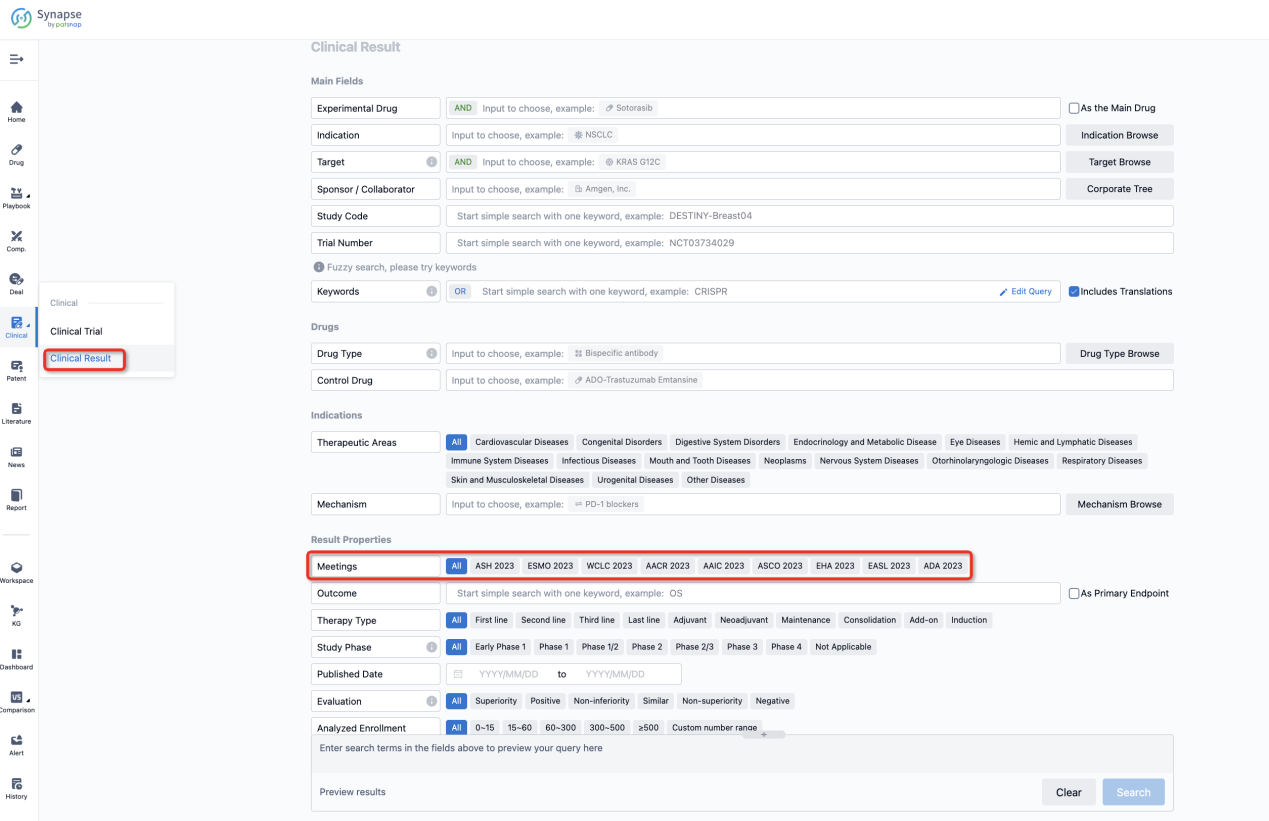
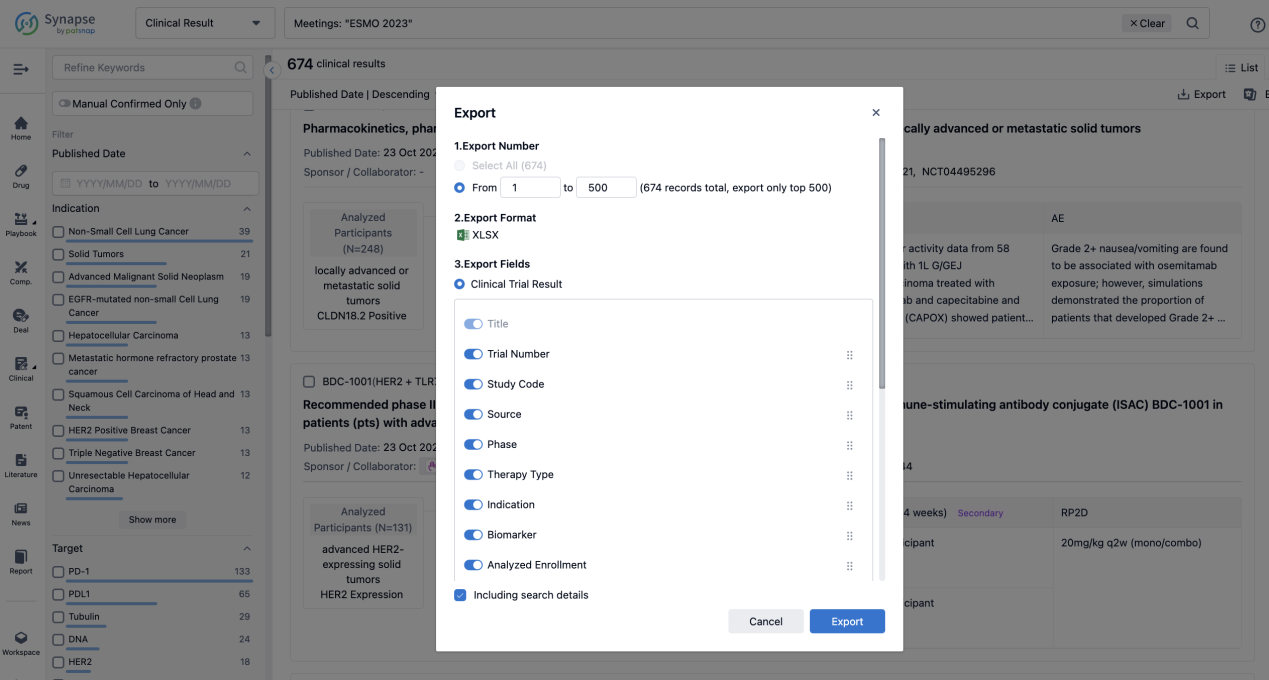
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
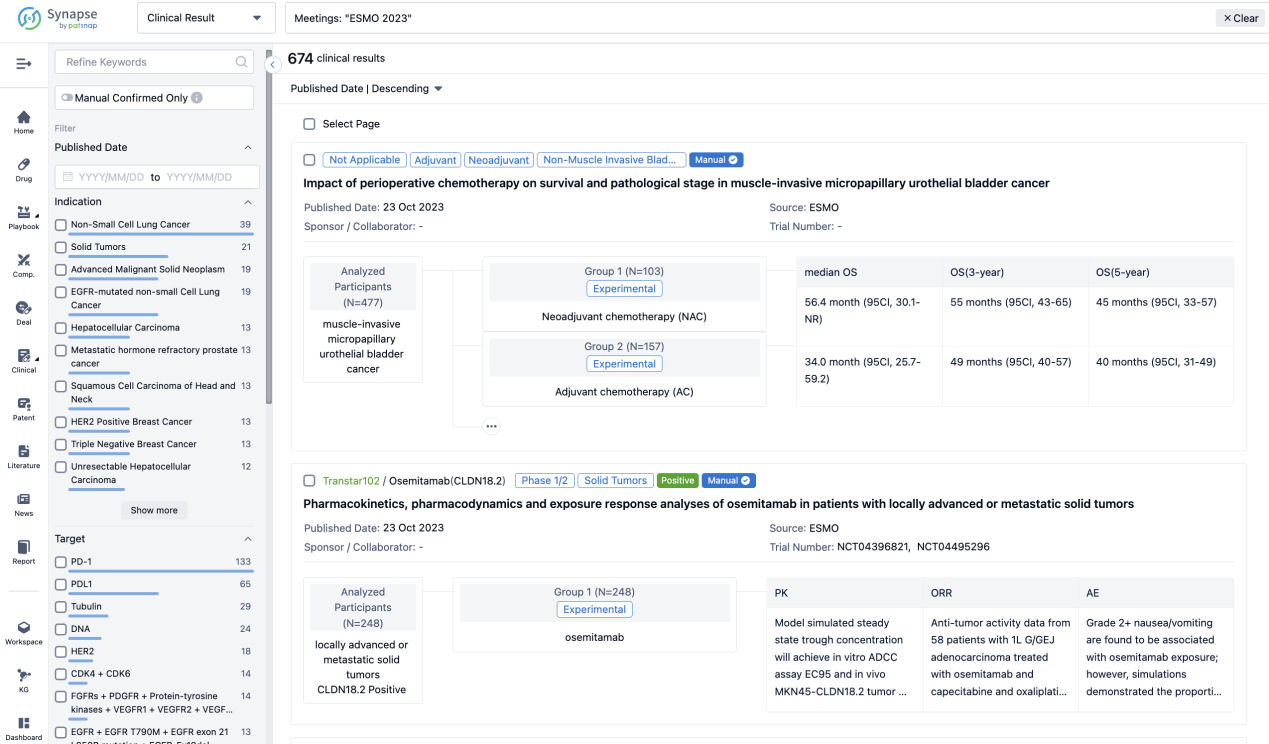
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
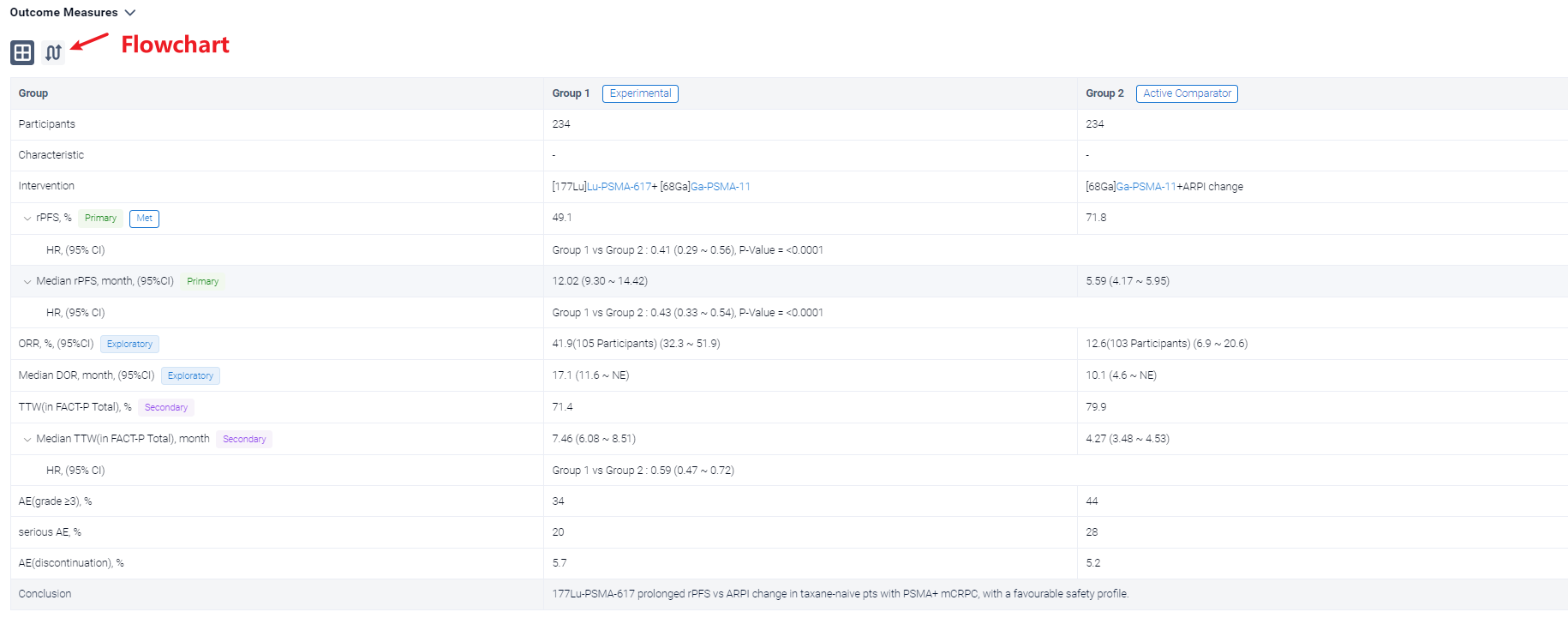
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!