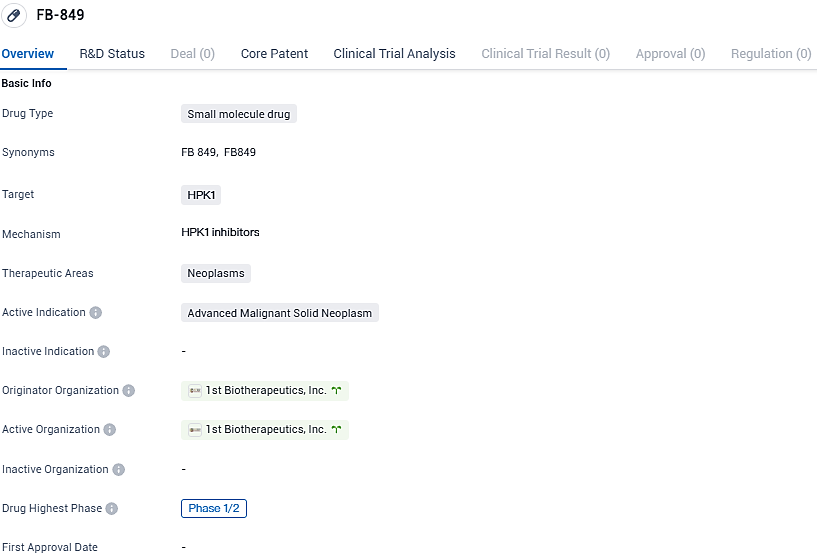
1ST Biotherapeutics, Inc. launches phase I/II trial of FB849 for progressive solid cancers
1ST Biotherapeutics, Inc., an enterprise specializing in the advancement of new pharmacological treatments operating in the clinical phase, predominantly in areas including neurodegenerative disorders, immuno-oncology, and uncommon health conditions, has officially commenced dosing the inaugural participant within its clinical Phase I/II study. This trial is designed to assess the efficacy of FB849 for individuals diagnosed with progressive solid neoplasms.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We are thrilled to share the commencement of our pioneering first-in-human study involving FB849 with the inaugural participant receiving treatment,” announced Seongkon Kim, Ph.D., Chief Technology Officer at 1ST Biotherapeutics. “FB849 is distinguishing itself as an advanced immunotherapeutic with remarkable specificity—yielding powerful responses against cancer and sustained benefits. This agent holds promise to fulfill significant unmet requirements in the field of immuno-oncology, offering benefits when used alone and when used synergistically with checkpoint inhibitors.”
The ongoing Phase I/II clinical trial is a non-blinded investigation aimed at ascertaining the safe usage, acceptance, pharmacological dynamics, and initial anti-tumor efficiency of FB849, both as a standalone therapy and when deployed in conjunction with KEYTRUDA, targeting advanced solid malignancies. 1ST Biotherapeutics is dedicated to the meticulous pursuit of clinical research excellence and eagerly anticipates providing further developments as the study moves forward.
1ST Biotherapeutics' innovative compound FB849 holds proprietary rights as a selective small molecule suppressor targeting hematopoietic progenitor kinase 1 (HPK1), signifying a frontier in immuno-oncological solutions. Preclinical discoveries have shown that through the targeted blockade of HPK1 by FB849, there is a notable amplification in anti-cancer immune function activating a range of immune cell types, which include T cells, B cells, dendritic cells, and macrophages. This action results in substantial and lasting repression of tumor proliferation.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
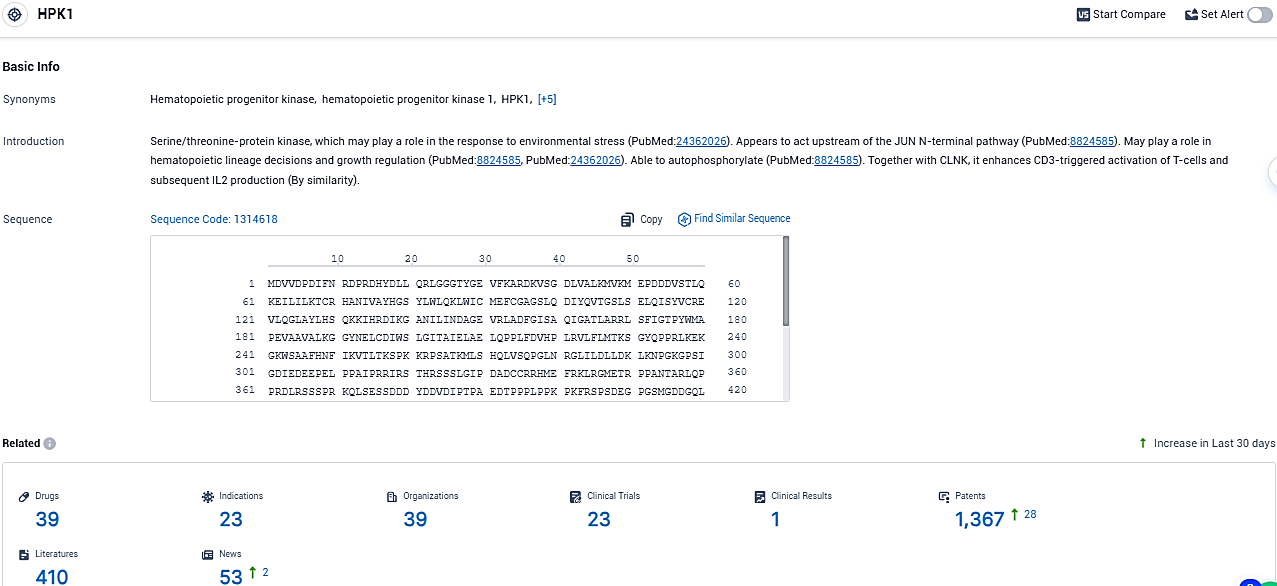 According to the data provided by the Synapse Database, As of December 21, 2023, there are 39 investigational drugs for the HPK1 target, including 23 indications, 39 R&D institutions involved, with related clinical trials reaching 23, and as many as 1367 patents.
According to the data provided by the Synapse Database, As of December 21, 2023, there are 39 investigational drugs for the HPK1 target, including 23 indications, 39 R&D institutions involved, with related clinical trials reaching 23, and as many as 1367 patents.
FB-849 is being developed for the treatment of advanced malignant solid neoplasms and is currently in Phase 1/2 of development. Further clinical trials will determine its potential as a therapeutic option for patients with late-stage.