Exploring TT-00434's R&D successes and its clinical results at the 2023 ESMO_ASIA
TT-00434 is an irreversible, highly selective inhibitor of FGFR1, 2, and 3 with potent preclinical activity against tumors harboring FGFR aberrations. The initial results from the first-in-human phase 1 study of TT-00434 orally administrated in patients (pts) with solid tumors were presented in 2023 ESMO_ASIA.
TT-00434's R&D Progress
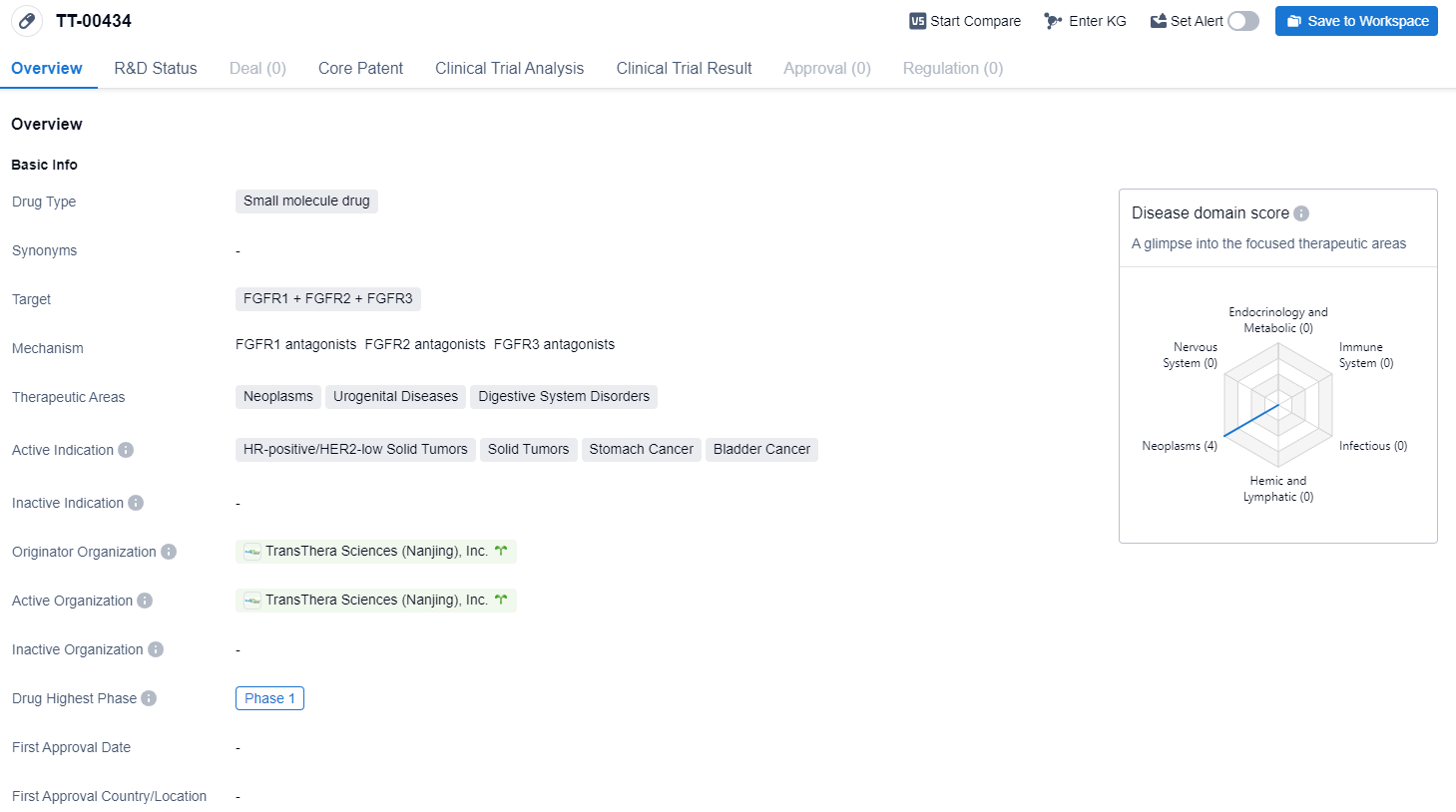
The drug TT-00434 is a small molecule drug that targets FGFR1, FGFR2, and FGFR3. It is primarily used in the treatment of neoplasms, urogenital diseases, and digestive system disorders. The active indications for this drug include HR-positive/HER2-low solid tumors, solid tumors, stomach cancer, and bladder cancer.
According to the Patsnap Synapse, TT-00434 is developed by TransThera Sciences (Nanjing), Inc., an originator organization in the pharmaceutical industry. Currently, the drug is in the highest phase of development, which is Phase 1 globally. And the clinical trial distributions for TT-00434 are primarily in China. The key indication is Advanced Malignant Solid Neoplasm. 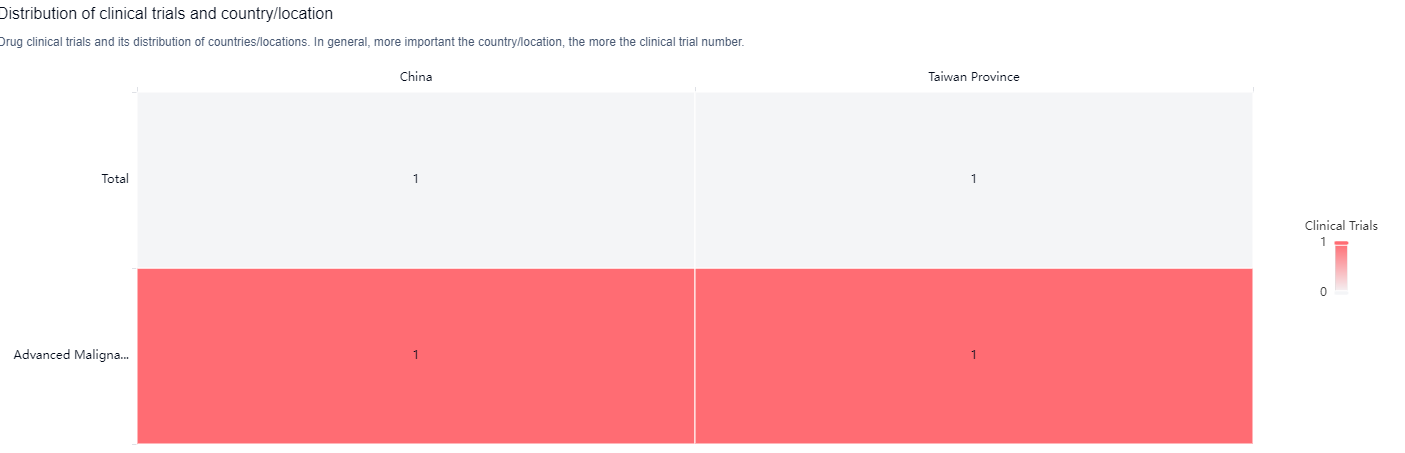
Detailed Clinical Result of TT-00434
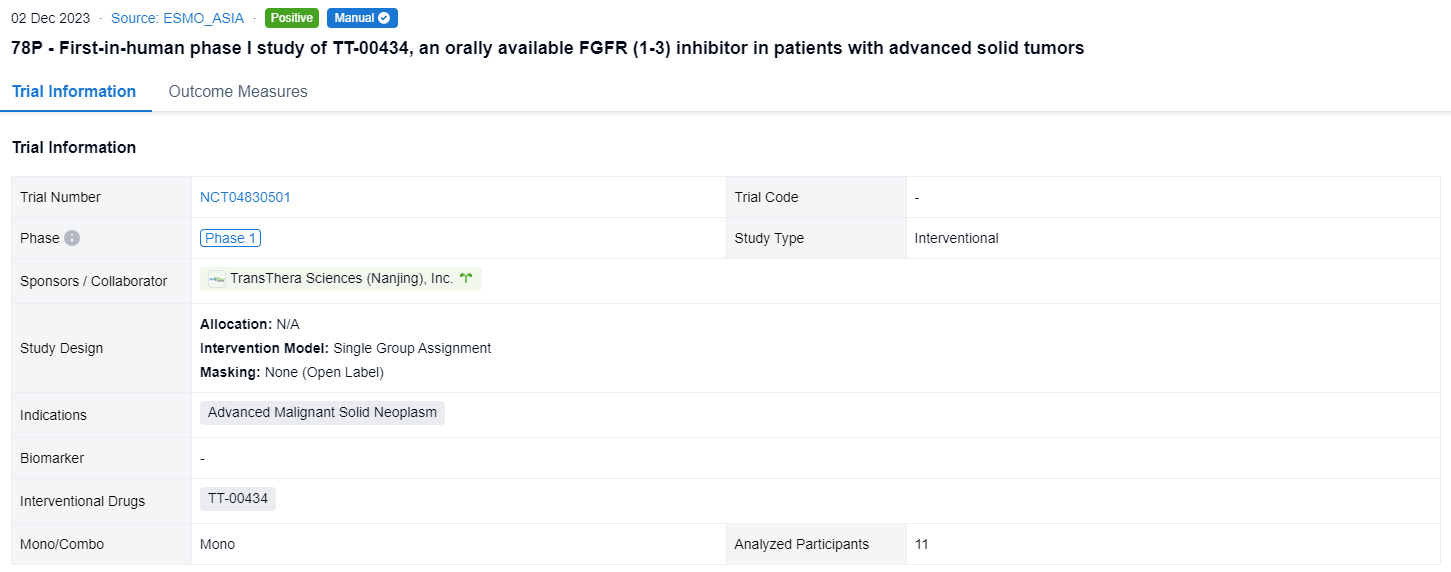
This single group assignment, open-labeled clinical trial (NCT04830501) was aimed to explore the preliminary antitumor activity and safety of TT-00434 in advanced solid tumors.
In this study, pts with advanced solid tumors who had exhausted all available standard treatments were enrolled. An accelerated titration design followed by a standard 3 + 3 scheme was used. Pts received a single daily dose of TT-00434 continuously for 28-day cycles. Key objectives included determining the recommended phase 2 dose (RP2D), safety, PK, pharmacodynamics (PD) and preliminary antitumor activity.
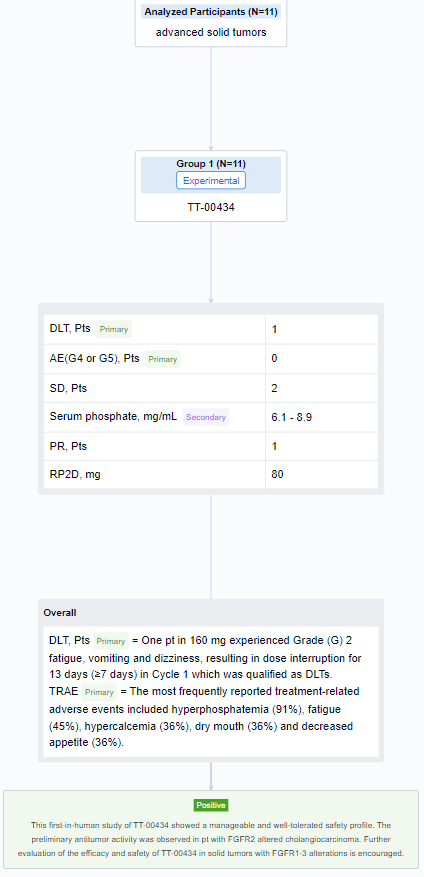
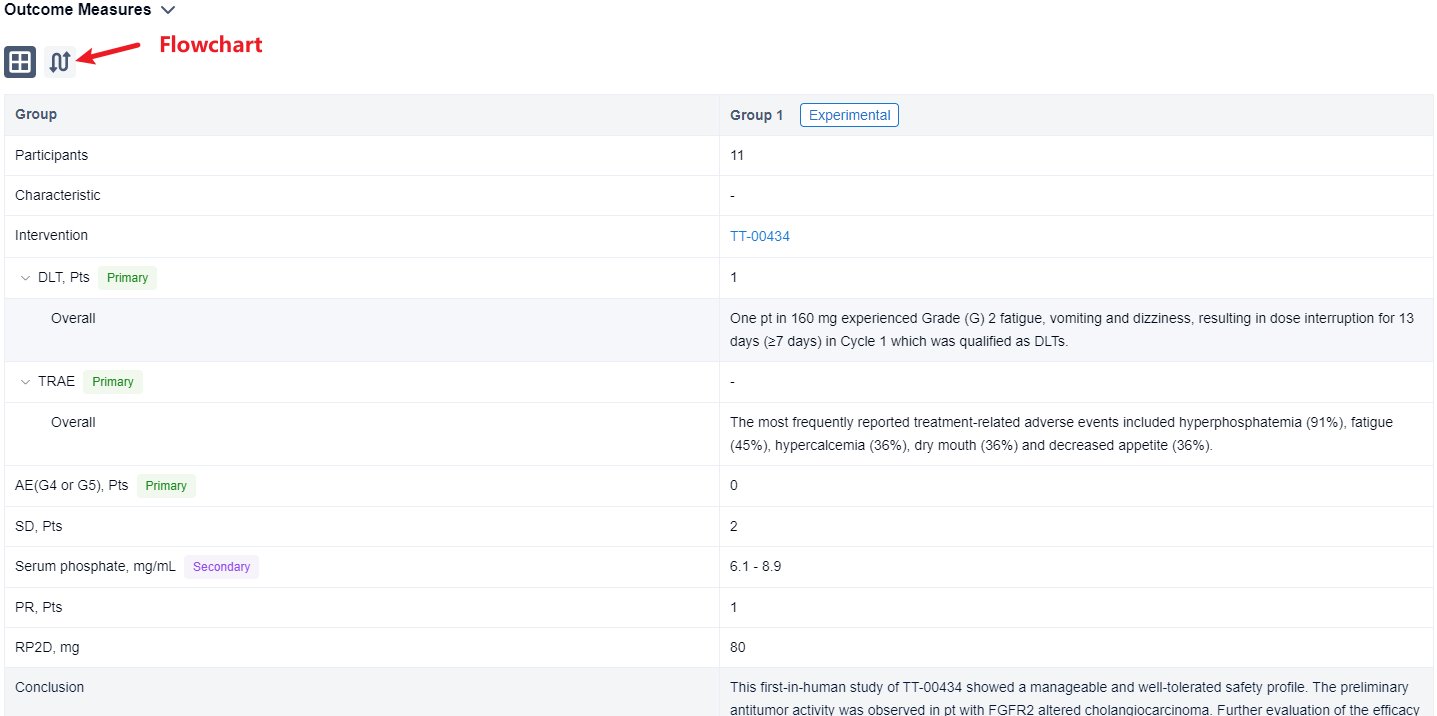
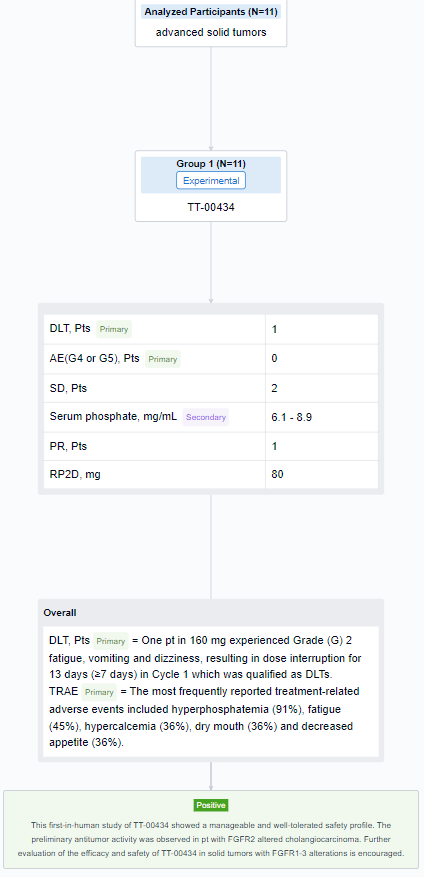
The result showed that as of 7 Aug 2023, 11 eligible pts received TT-00434 40 mg (n = 1), 80 mg (n = 6), and 160 mg (n = 4). One pt in 160 mg experienced Grade (G) 2 fatigue, vomiting and dizziness, resulting in dose interruption for 13 days (≥7 days) in Cycle 1 which was qualified as DLTs. The most frequently reported treatment-related adverse events included hyperphosphatemia (91%), fatigue (45%), hypercalcemia (36%), dry mouth (36%) and decreased appetite (36%). Two G3 events of fatigue and hyponatremia (each n=1) were observed. No G4 or G5 AEs were reported. The exposure (AUC and Cmax) was dose proportional from 80 to 160 mg. The maximum serum phosphate concentration as PD biomarker reached plateau (6.1∼8.9 mg/mL) at 80 mg. Stable diseases were observed in 2 pts with bladder cancer and endometrial cancer. One cholangiocarcinoma pt with FGFR alteration (FGFR2(17)-ARHGAP24(3) fusion) treated at 80 mg achieved partial response. The treatment lasts 203+ days and is still ongoing. Based on safety, efficacy and PK/PD assessments, the RP2D was determined as 80 mg QD.
It can be concluded that this first-in-human study of TT-00434 showed a manageable and well-tolerated safety profile. The preliminary antitumor activity was observed in pt with FGFR2 altered cholangiocarcinoma. Further evaluation of the efficacy and safety of TT-00434 in solid tumors with FGFR1-3 alterations is encouraged.
How to Easily View the Clinical Results Using Synapse Database?
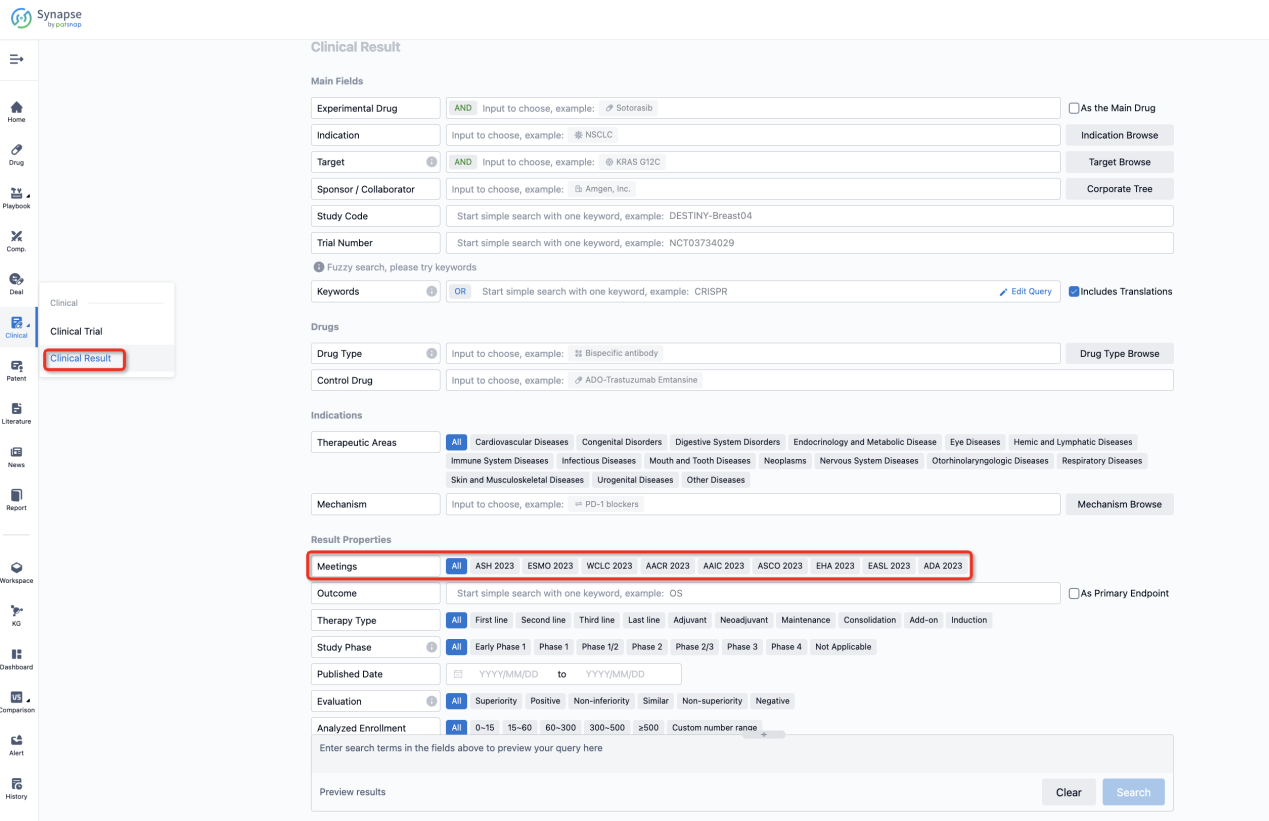
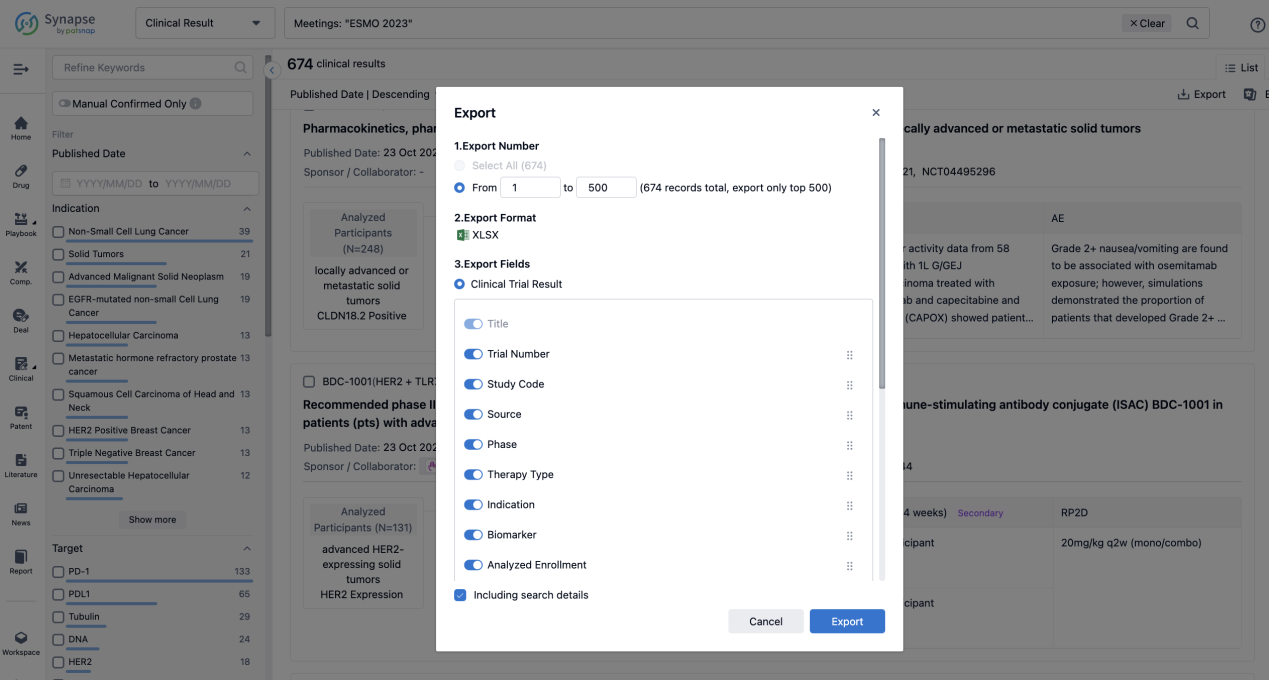
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
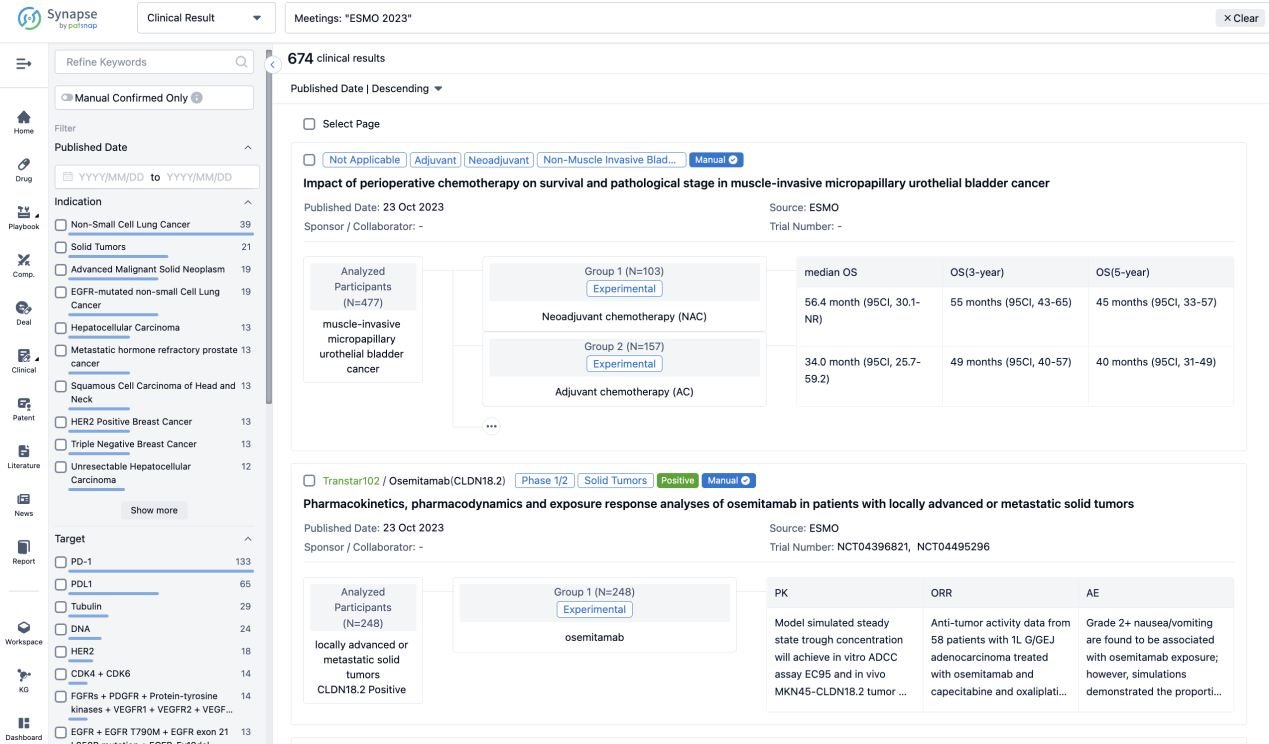
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!