Shattuck Labs reports encouraging initial outcomes from ongoing Phase 1 A/B trial of SL-172154 and Azacitidine in advanced, newly diagnosed HR-MDS and TP53-mutated AML cases
Shattuck Labs, Inc., an innovative firm at the clinical-stage forefront in the biotech industry, is advancing the creation of dual-functional fusion proteins, emerging as a novel category of biological therapeutics. These innovative drugs are designed to serve patients battling oncological and autoimmune conditions. The company recently disclosed preliminary aggregate data from an advancing stage of their Phase 1A/B clinical investigation. This trial is examining the combined effects of SL-172154 and AZA as a frontline treatment for individuals diagnosed with high-risk myelodysplastic syndromes (HR-MDS) and acute myeloid leukemia with TP53 mutations (TP53m AML).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Following the middle-year conclusion of our dose-escalation research, the recruitment for both HR-MDS and TP53 mutant AML specific cohorts advanced rapidly. "We're excited to divulge some preliminary outcomes illustrating the promising performance of SL-172154, which appears to surpass the anticipated effects of AZA monotherapy," announced Dr. Lini Pandite, MBChB, M.B.A., the Chief Medical Officer at Shattuck.
In the initial phase, the observed rate of complete remissions in HR-MDS and TP53 mutant AML groups has been quite positive. Moreover, noting the hematologic improvement in several patients not yet achieving complete remission, it's inferred that the rate of complete remissions could further increase with time. Consequently, modifications have been made to both clinical trials to expand the participant numbers, and we anticipate an additional progress report around the mid of 2024.
The presentation given at ASH, entitled “Safety, Pharmacodynamic, and Anti-Tumor Activity of SL-172154 as Monotherapy and in Combination with Azacitidine in Relapsed/Refractory Acute Myeloid Leukemia and Higher-Risk Myelodysplastic Syndromes/Neoplasms Patients,” can be found on Shattuck’s website in the “Our Science” segment, beneath the posters category.
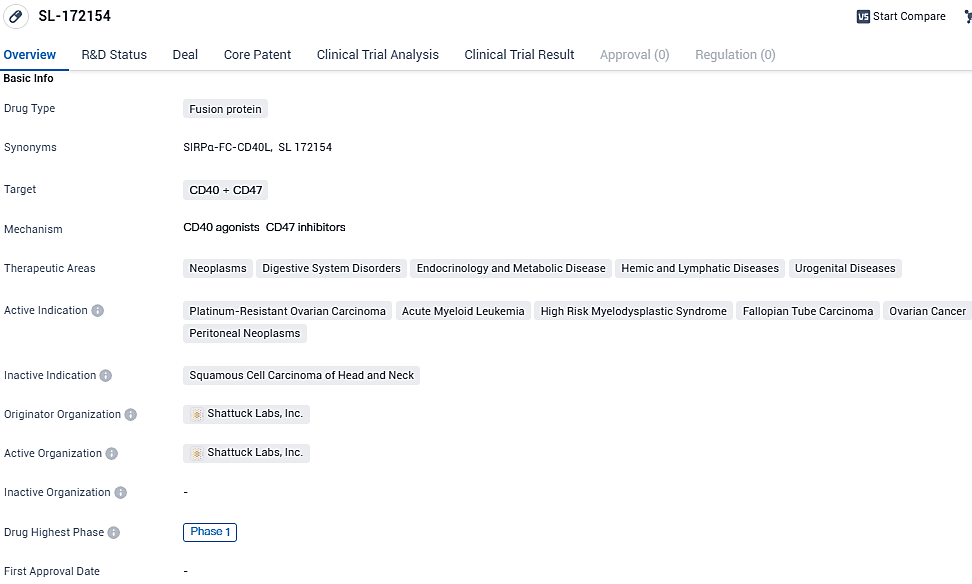
SL-172154 (SIRPα-Fc-CD40L) is a novel trial ARC® fusion protein targeting to concurrently thwart the CD47/SIRPα interaction and stimulate CD40, thereby promoting an immune response against advanced stage cancers. There are several Phase 1 clinical trials in progress focusing on patients diagnosed with PROC as well as those suffering from AML and HR-MDS.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
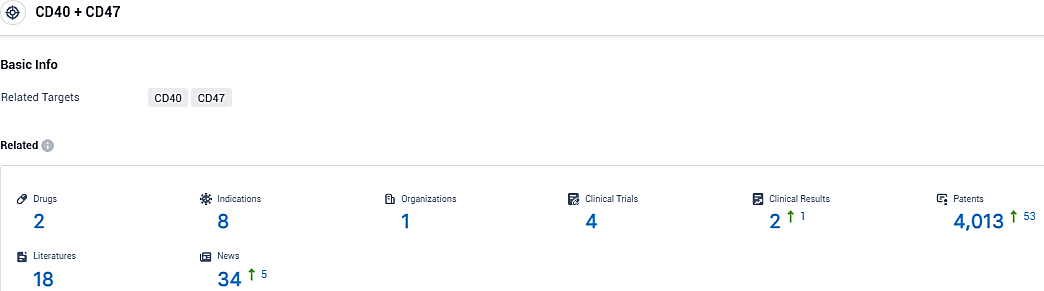
According to the data provided by the Synapse Database, As of December 21, 2023, there are 2 investigational drugs for the CD40 and CD47 target, including 8 indications, 1 R&D institutions involved, with related clinical trials reaching 4, and as many as 4013 patents.
The active indications for SL-172154 include platinum-resistant ovarian carcinoma, acute myeloid leukemia, high-risk myelodysplastic syndrome, fallopian tube carcinoma, ovarian cancer, and peritoneal neoplasms. These indications indicate that SL-172154 may be effective in treating these specific types of cancers and related conditions.