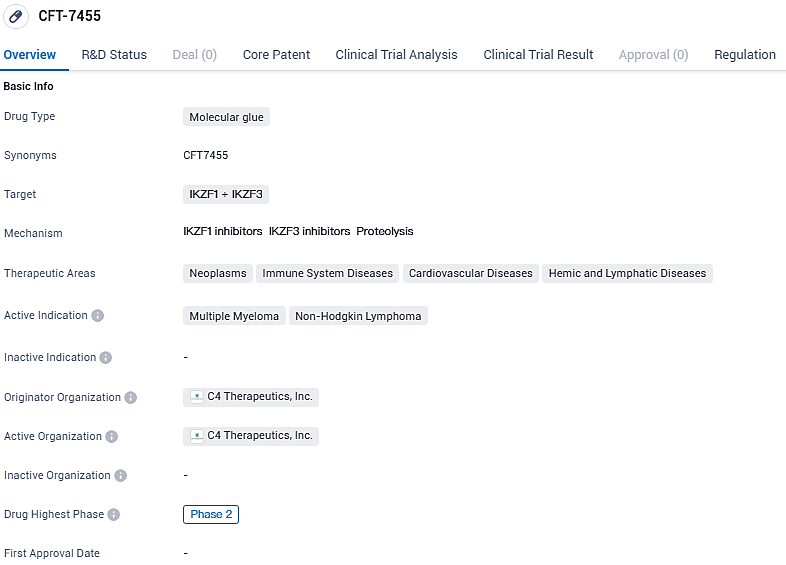
C4 Therapeutics Reports Encouraging Results from Initial Study of CFT7455 in Recurrent/Resistant Multiple Myeloma Cases
C4 Therapeutics, Inc., an enterprise in the phase of clinical development focusing on the progression of selective protein degradation technology for the creation of innovative small-molecule drugs, has unveiled clinical findings from the escalating dose stage in the first phase of its Phase 1/2 study. The ongoing trial features CFT7455, a distinctive MonoDAC™ agent engineered to dismantle IKZF1/3 proteins, with the aim of potentially addressing conditions such as multiple myeloma and non-Hodgkin lymphomas.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The dataset consists of findings from CFT7455 administered alone in patients with relapsed or refractory multiple myeloma (MM), where the phase of increasing dosage levels has been concluded, as well as preliminary outcomes from tests of CFT7455 used in tandem with dexamethasone to treat patients with R/R MM who are still being recruited. Patient recruitment for the initial Phase 1 study assessing CFT7455 alone for those with non-Hodgkin lymphoma (NHL) is also ongoing.
"CFT7455, as a standalone treatment, is demonstrating encouraging evidence of activity against myeloma cells and capability to modulate the immune system and when paired with dexamethasone, it seems particularly effective for individuals who have tried several treatment lines for multiple myeloma, including therapies targeting BCMA," Len Reyno, M.D., the Chief Medical Officer at C4 Therapeutics, expressed his optimism.
Dr. Reyno further explained, "We have pinpointed a 14-day on/off administration pattern as the most effective regimen, in harmony with our lab findings. This supports CFT7455 as a thoughtfully developed IKZF1/3 inhibitor, which may provide a novel therapeutic option for patients dealing with relapsed/refractory multiple myeloma."
The primary aim of the ongoing Phase 1 increase in dose research for CFT7455 is to establish its safety parameters, figure out the highest dose that can be tolerated or given, and recognize any indications of its capacity to counteract tumor growth in patients with R/R MM or R/R NHL.
The dose-escalation segment of this Phase 1 research is tripartite, consisting of: CFT7455 given alone for R/R MM patients, which is already complete; the CFT7455 and dexamethasone combination for R/R MM patients, moving forward in dosage increments; and CFT7455 as a single treatment for NHL patients, which is also progressing through its dosage increase phase.
Dose escalation for the monotherapy arm has been finished. By the November 28, 2023 data cutoff, CFT7455 had been administered alone to 22 individuals. The highest dose provided was 75 micrograms on a biweekly cycle, 14 days on followed by 14 days off. A maximum tolerated dose hasn't been specified. The median number of prior treatments among the subjects was seven, with most having been treated previously with CAR-T or T-cell engaging therapies.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
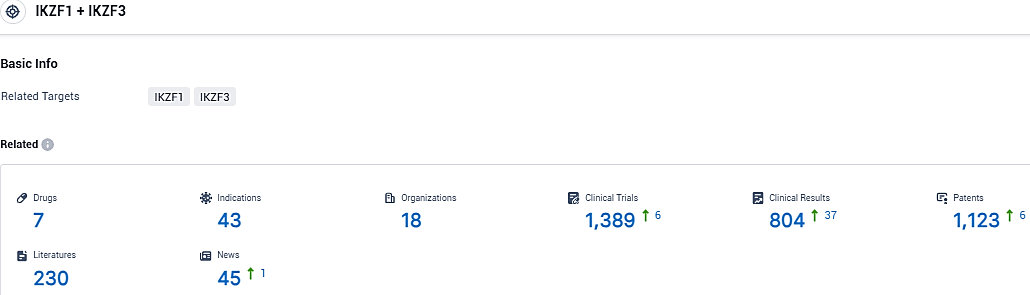
According to the data provided by the Synapse Database, As of December 21, 2023, there are 7 investigational drugs for the IKZF1/3 target, including 43 indications, 18 R&D institutions involved, with related clinical trials reaching 1389, and as many as 1123 patents.
CFT7455 is an orally bioavailable MonoDAC™ degrader designed to be highly potent and selective against its intended targets of Ikaros and Aiolos and overcome shortcomings of currently approved therapies to treat multiple myeloma and non-Hodgkin’s lymphoma. The drug has reached Phase 2 of clinical trials and has been designated as an orphan drug, indicating its potential to address unmet medical needs in rare diseases.