Analysis on the Clinical Research Progress of FcRn Antagonists
FcRn, or neonatal Fc receptor, is a crucial protein found in the human body that plays a significant role in the immune system and the pharmacokinetics of therapeutic antibodies.
The neonatal Fc receptor (FcRn) is a major histocompatibility complex (MHC) class I molecule, consisting of an α chain encoded by the FCGRT (Fc fragment of IgG receptor and transporter) gene and a β-2-microglobulin (β2m). The primary role of FcRn is to bind, transport, and recycle immunoglobulin G (IgG) and albumin, thereby protecting them from lysosomal degradation. Due to this selective recycling, IgG has a longer serum half-life compared to other antibody classes. Although FcRn is referred to as the "neonatal" Fc receptor, it is expressed throughout a person's lifespan and is widespread across various tissues and cells. FcRn is primarily expressed in endothelial cells (particularly in the large vascular beds of the skin and muscle, and in the liver) and antigen-presenting cells (macrophages/monocytes, DC cells, and B cells, etc.). Moreover, FcRn is also expressed on certain mucosal surfaces and in epithelial cells, such as intestinal epithelial cells, bronchial and alveolar epithelial cells, etc.
Understanding the role of FcRn is essential for the development of antibody-based therapies and optimizing their pharmacological properties.
FcRn Competitive Landscape
According to Patsnap Synapse, as of 13 Oct 2023, there are a total of 12 FcRn drugs worldwide, from 35 organizations, covering 28 indications, and conducting 208 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.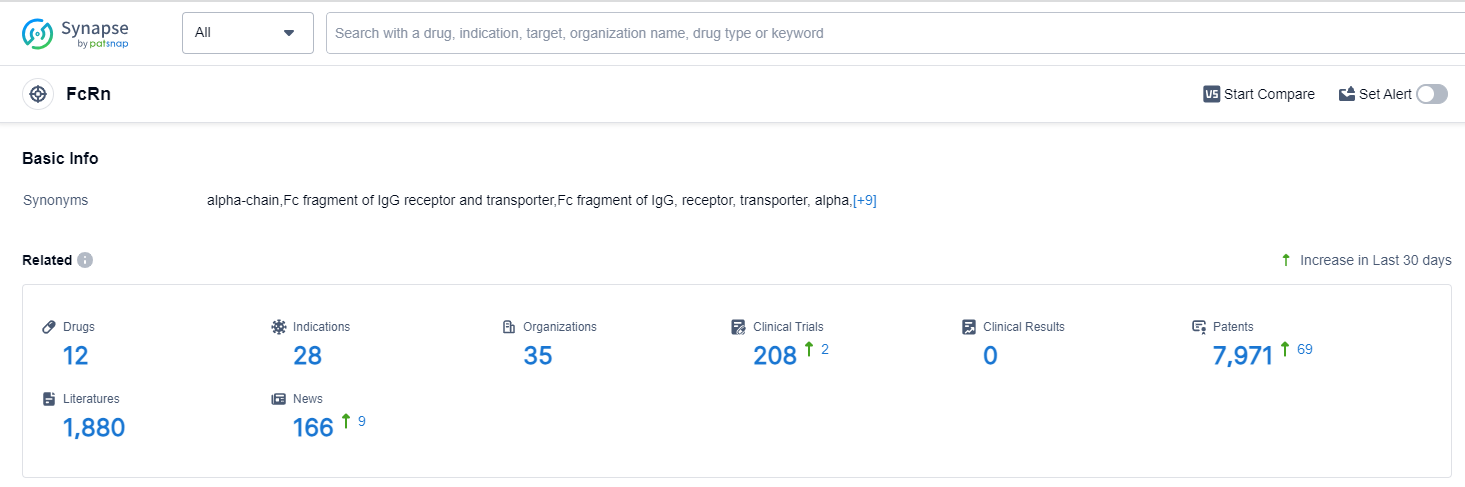
The analysis of the current competitive landscape of target FcRn reveals that companies like argenx SE, UCB SA, and Roivant Sciences Ltd. are leading in terms of R&D progress. These companies have a significant number of drugs in various stages of development, indicating their focus on advancing drugs targeting FcRn. The approved drugs under the target FcRn have indications covering a wide range of autoimmune and hematological disorders, highlighting the potential of these drugs in treating these conditions. The presence of biosimilars, such as Fc Fragment, indicates intense competition around the innovative drugs targeting FcRn. The countries/locations developing fastest under the current target FcRn are the United States, Japan, and the European Union, with China also showing progress. The future development of target FcRn is expected to be driven by the continued R&D efforts of these companies, the expansion of indications for approved drugs, and the competition among different drug types and countries/locations.
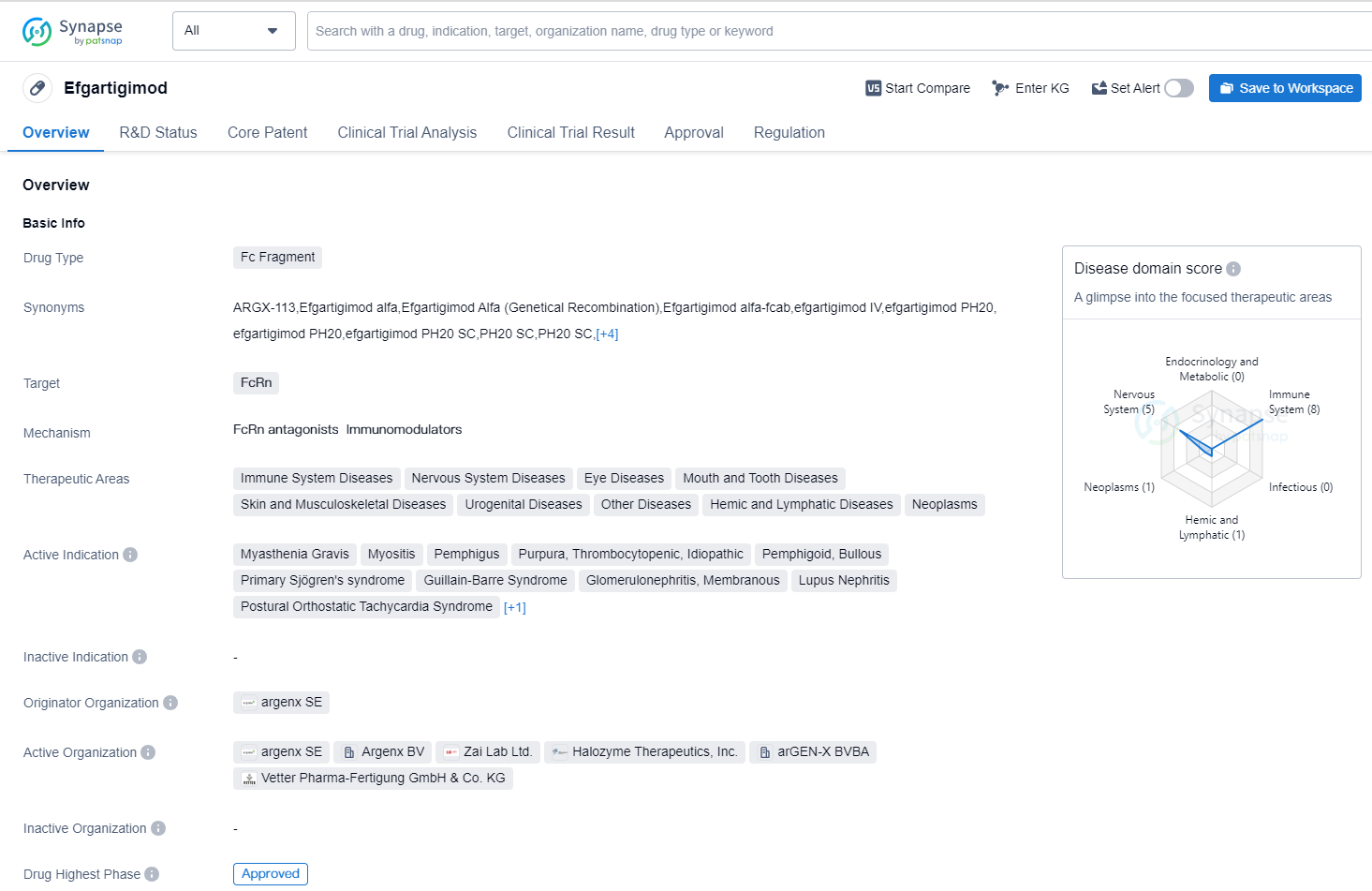
Key drug:Efgartigimod
Efgartigimod is a drug classified as an Fc Fragment, which targets FcRn. It is primarily used in the treatment of various immune system diseases, nervous system diseases, eye diseases, mouth and tooth diseases, skin and musculoskeletal diseases, urogenital diseases, other diseases, hemic and lymphatic diseases, and neoplasms. The active indications for efgartigimod include Myasthenia Gravis, Myositis, Pemphigus, Purpura Thrombocytopenic Idiopathic, Pemphigoid Bullous, Primary Sjögren's syndrome, Guillain-Barre Syndrome, Glomerulonephritis Membranous, Lupus Nephritis, Postural Orthostatic Tachycardia Syndrome, and Polyradiculoneuropathy Chronic Inflammatory Demyelinating.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Efgartigimod was developed by argenx SE and has received approval for use in the United States, with the first approval date recorded as December 2021. It is worth noting that efgartigimod has also obtained approval in China. The drug has reached the highest phase of development, with global approval.
In terms of regulatory status, efgartigimod has been granted several designations, including Orphan Drug, Fast Track, Priority Review, Promising Innovative Medicine, Paediatric investigation plan, and Breakthrough Therapy. These designations highlight the potential significance and urgency of the drug in addressing unmet medical needs.
As an Fc Fragment targeting FcRn, efgartigimod holds promise in the treatment of a wide range of diseases affecting different systems of the body. Its approval in both the United States and China indicates its potential global impact. The regulatory designations it has received further emphasize its potential as a breakthrough therapy.
Overall, efgartigimod represents a significant advancement in the field of biomedicine, particularly in the treatment of immune system diseases and other related conditions. Its approval and regulatory designations highlight its potential to address unmet medical needs and improve patient outcomes.
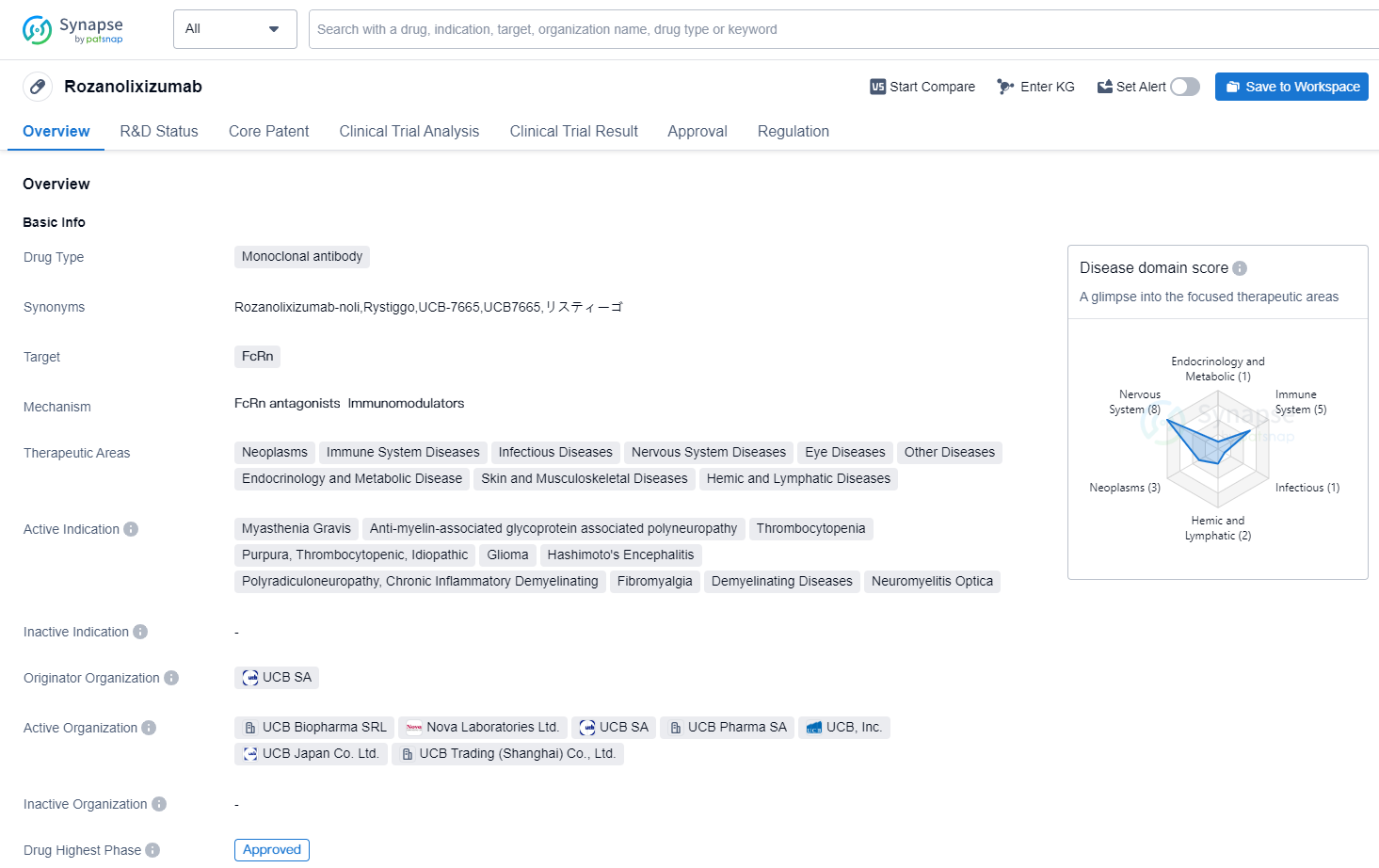
Rozanolixizumab
Rozanolixizumab is a monoclonal antibody drug that targets FcRn, a protein involved in the recycling of immunoglobulins. It has shown potential therapeutic benefits in various therapeutic areas, including neoplasms, immune system diseases, infectious diseases, nervous system diseases, eye diseases, endocrinology and metabolic diseases, skin and musculoskeletal diseases, and hemic and lymphatic diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has been indicated for the treatment of several conditions, including myasthenia gravis, anti-myelin-associated glycoprotein associated polyneuropathy, thrombocytopenia, purpura, thrombocytopenic, idiopathic, glioma, Hashimoto's encephalitis, chronic inflammatory demyelinating polyradiculoneuropathy, fibromyalgia, demyelinating diseases, and neuromyelitis optica.
Rozanolixizumab was developed by UCB SA, a pharmaceutical company specializing in the research and development of innovative therapies. It has reached the highest phase of development, with global approval expected in June 2023. In China, the drug is currently in phase 3 of clinical trials.
The first approval of Rozanolixizumab is anticipated to be granted in the United States. The drug has been designated for priority review, indicating its potential to address unmet medical needs. Additionally, it has received orphan drug status, which provides incentives for the development of drugs targeting rare diseases.
Overall, Rozanolixizumab shows promise as a therapeutic option for a wide range of diseases and conditions. Its mechanism of action, targeting FcRn, suggests potential benefits in modulating the immune system and addressing various pathological processes. The drug's progress through clinical trials and its upcoming global approval highlight its potential to make a significant impact in the field of biomedicine.