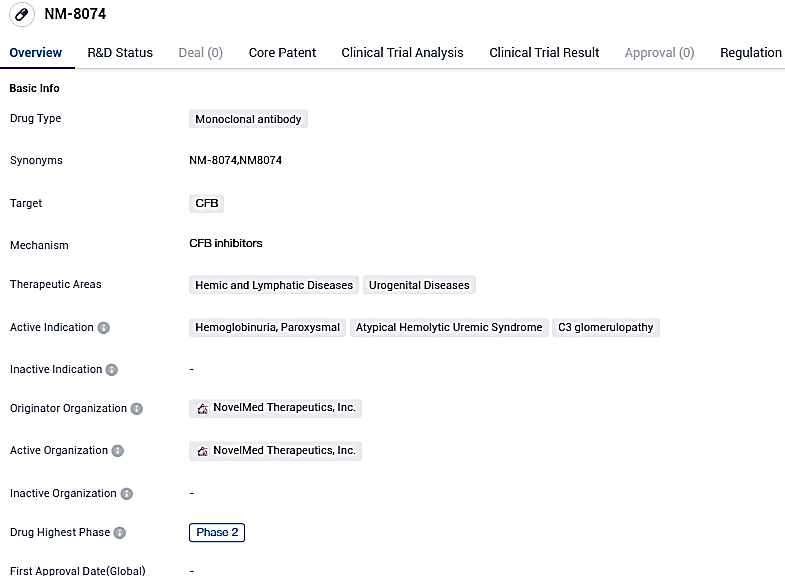
NovelMed Initiates Phase II Trial of Anti-Bb Antibody (NM8074) for Untried PNH Patients: An Insight into the PNH Study Development
NovelMed Therapeutics is thrilled to declare the initiation of its Phase II clinical trial, the objective of which is to explore treatment opportunities for Paroxysmal Nocturnal Hemoglobinuria patients who are yet untreated. The focus measure, a pioneering anti-Bb antibody called NM8074, will be tested in an open-label, multi-dose, and multi-center study. The objective of this investigation is to evaluate NM8074's security and effectiveness in the PNH patient cluster.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
So far, eight participants diagnosed with PNH have been included in the study, with two already undergoing NM8074 therapy. Crucial parameters for effectiveness comprise augmenting Hemoglobin, RBC clone size enlargement, reducing Lactate Dehydrogenase quantities, lessening pRBC transfusions, and decreasing D-Dimer quantities. Attaining positive results in these aspects could establish NM8074 as a distinctive and pioneering biologic for first-time PNH patients.
The Phase II trial is divided into two segments, utilizing a fortnightly dosing routine over an evaluation period of three months. The first impressions from data indicate that NM8074 manifests a pleasing safety record, devoid of any safety issues. NM8074 delivered treatment to fresh PNH patients whose clone size was more than 10%.
The findings show considerable deductions in LDH, Reticulocytes, Free Hemoglobin, and D-dimer, together with surges in Hemoglobin and Haptoglobin quantities as envisaged. Additionally, the clone size proliferated in alignment with time. In conclusion, the observed modifications in efficacy parameters confirm the implications of the drug's functionality and support the role of NM8074 as a viable PNH treatment.
Dr. Rekha Bansal, CEO of NovelMed Therapeutics, remarked, "Our early data from the Phase II study in first-time PNH patients affirms the potential of NM8074 to holistically manage intravascular and extravascular anemia caused by hemolysis and fortify the patient's defense mechanisms, hence minimizing infection risk."
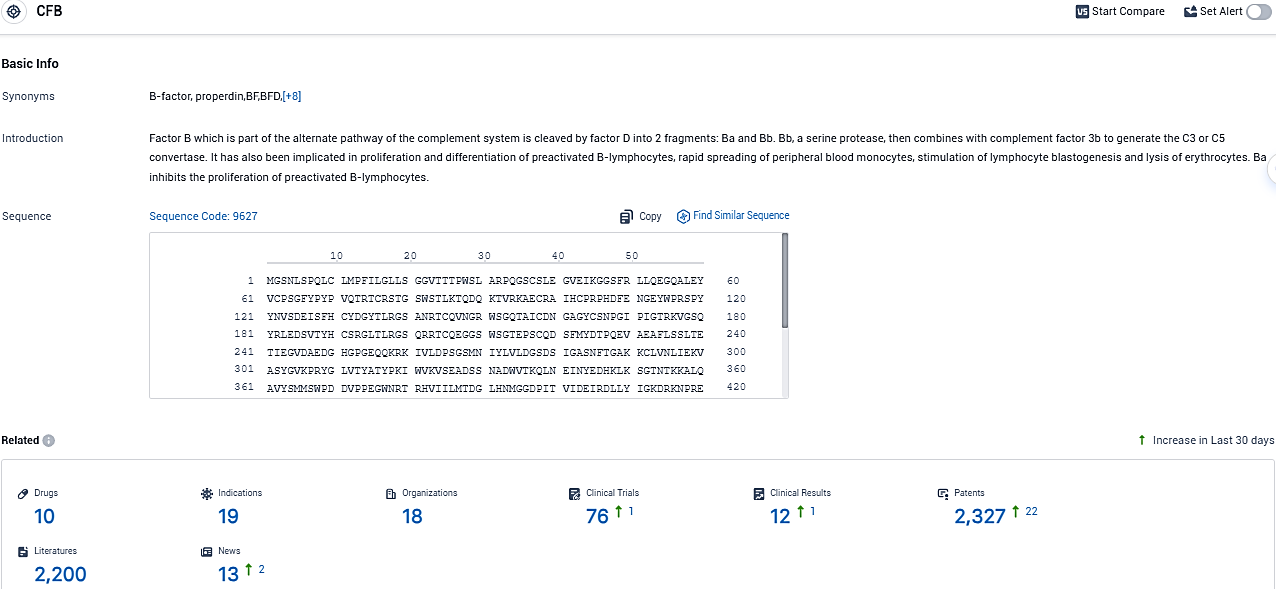
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 2, 2023, there are 10 investigational drugs for the CFB target, including 19 indications, 18 R&D institutions involved, with related clinical trials reaching 76, and as many as 2200 patents.
NM8074 stands out as a monoclonal antibody characterized by its dual specificity, which distinguishes it from medical alternatives like Iptacopan that latch onto Factor B. It zeroes in on Bb and specifically inhibits the alternative pathway. Unlike other third-party complement blockers, NM8074 presents a special action process. It is anticipated to be a superior treatment solution, possibly exempt from the Black-Box warning that current FDA-endorsed treatments carry.