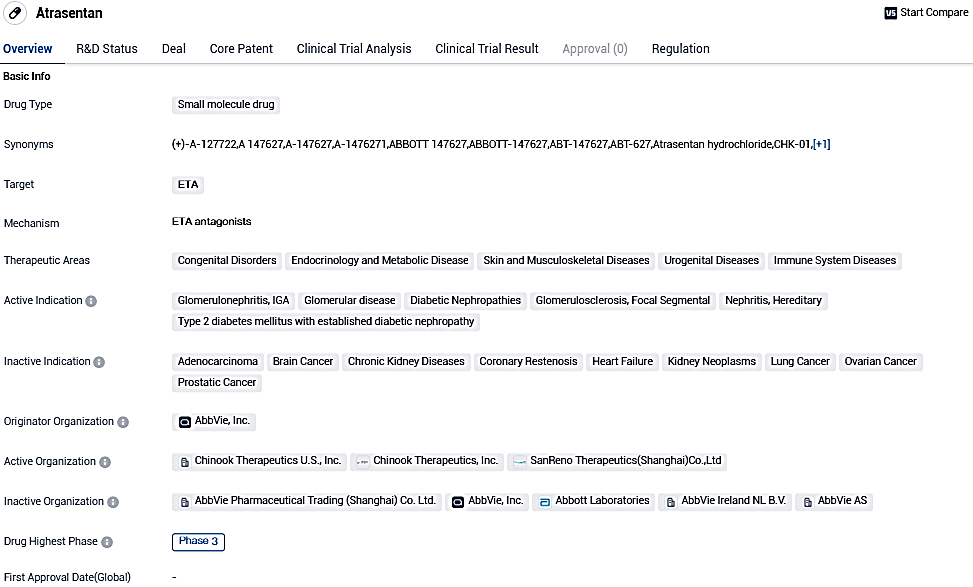
Novartis's atrasentan trial in Phase III showed effective and notable proteinuria reduction in IgA nephropathy (IgAN) patients with high statistical significance
Novartis has released favourable preliminary findings from the midpoint evaluation of the pivotal Phase III ALIGN trial, which is studying atrasentan, an oral endothelin A receptor antagonist (ERA), in individuals suffering from IgA nephropathy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The primary efficiency endpoint of the study was achieved at the 36-week interim analysis, indicating atrasentan's superiority over placebo through a clinically significant and highly statistical reduction of proteinuria in IgAN patients under supportive care.
The safety of atrasentan was found to be congruent with previously reported data from the IgAN cohort in the AFFINITY Phase II study. In light of the interim proteinuria endpoint analysis results, Novartis intends to apply for possible expedited approval in the US in 2024.
"Encouraging preliminary results from Phase III underscore the capacity of atrasentan to potentially enhance outcomes for IgAN patients by demonstrating significant proteinuria reduction," said Shreeram Aradhye, M.D., President of Development, and Chief Medical Officer at Novartis.
"Moreover, alongside the investigational iptacopan which recently exhibited promising Phase III findings, and zigakibart under investigation, our portfolio with three diverse late-stage treatments for IgAN may provide much-needed therapeutic options for those suffering from this incapacitating disease," added Shreeram Aradhye.
Atrasentan, an oral endothelin A receptor antagonist under investigation for IgAN and other uncommon kidney diseases, was included in Novartis' portfolio following the purchase of Chinook Therapeutics, and also includes zigakibart under investigation, a subcutaneously injected anti-APRIL monoclonal antibody in Phase III development for IgAN.
Novartis expanded its kidney-related portfolio with the addition of these two therapies along with an early-stage pipeline, which also features iptacopan, a factor B inhibitor under investigation that recently yielded positive interim results from Phase III in IgAN. To address unfilled gaps in IgAN and other infrequent kidney disorders, Novartis is pushing forward with the development of these three potential treatment alternatives, each having different action mechanisms.
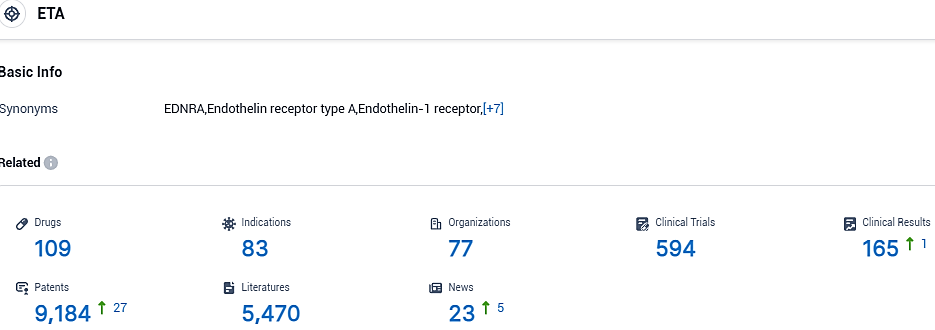
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 1, 2023, there are 109 investigational drugs for the ETA target, including 83 indications, 77 R&D institutions involved, with related clinical trials reaching 594, and as many as 9184 patents.
Atrasentan, an oral endothelin A receptor antagonist (ERA) currently under investigation, is undergoing Phase III trials for IgAN and preliminary trials for other uncommon kidney diseases. In a Phase II trial for IgAN, Atrasentan demonstrated prominent decreases in proteinuria compared to the baseline.