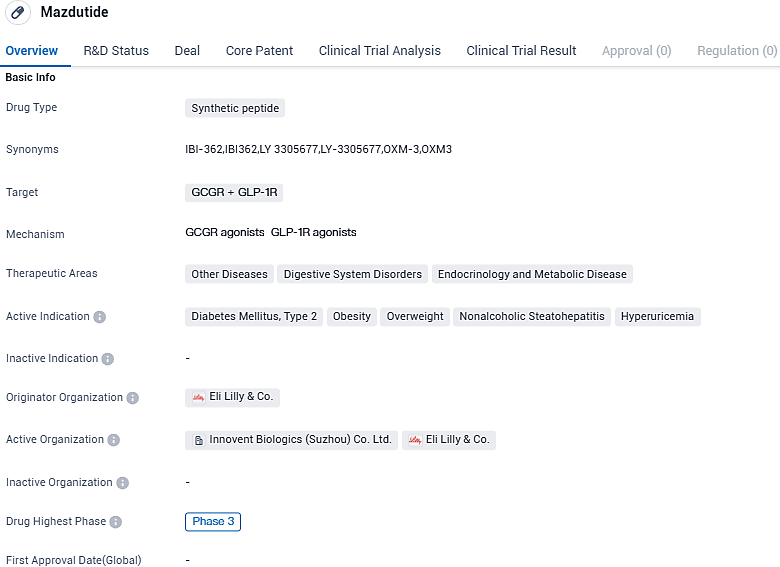
Innovent reports significant weight loss, multiple metabolic benefits, and positive safety record with a higher 9mg dose of Mazdutide (IBI362), after a 48-week Phase 2 trial for obesity
Innovent Biologics, Inc., a globally esteemed biotech company focused on the creation, production, and sales of exceptional remedies for major diseases such as cancer, metabolic, autoimmune, eye-related ailments and more, has made known their 48-week therapy outcomes from a phase 2 clinical trial involving a greater dosage (9 mg) of mazdutide on obese Chinese individuals. The results showcased remarkable efficiency in weight reduction, beneficial safety implications, and various metabolic advantages.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The findings indicate that mazdutide 9 mg could potentially be an alternate solution to metabolic surgery for the long-term weight control in Chinese people suffering from moderate to severe obesity. Innovent has plans to commence the Phase 3 clinical trial of mazdutide 9 mg in Chinese patients diagnosed with obesity by the conclusion of 2023.
The Phase 2 trial is a double-blind, randomized, placebo-controlled study aiming to assess the efficacy and safety of a larger dose of 9mg mazdutide in overweight Chinese subjects with an average initial body mass index (BMI) of 34.3 kg/m2. About 80 subjects were enrolled and allocated to either mazdutide 9 mg or a placebo in a 3:1 ratio.
The primary objective of this research study is the percentage fluctuation in body weight from the initial measure compared to the placebo after 24 weeks of treatment. The research was also extended to 48 weeks for participants who consented to receive an additional double-blind extension treatment for 24 weeks.
Professor Linong Ji, the senior investigator of the research from Peking University People's Hospital, stated that, "Obesity, a chronic illness with multiple underlying causes, poses a significant risk factor for metabolic, cardiovascular and cerebrovascular diseases and cancer. Obesity needs long-term care and management, plus society's full attention. I am eagerly anticipating the Phase 3 clinical trial of mazdutide 9 mg in Chinese patients suffering from obesity and its future implementation in clinical settings."
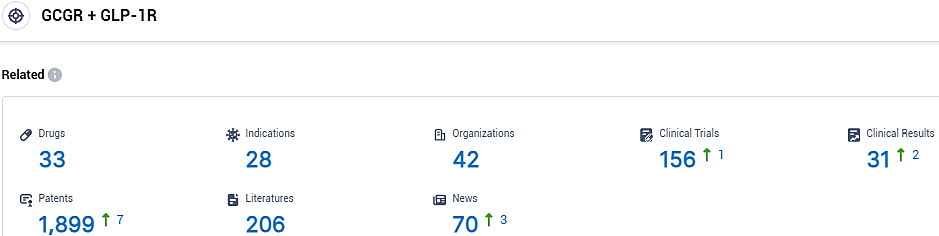
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 1, 2023, there are 33 investigational drugs for the GCGR and GLP-1R target, including 28 indications, 42 R&D institutions involved, with related clinical trials reaching 156, and as many as 1899 patents.
Trials have shown that Mazdutide delivers efficient weight reduction and glucose management results, while decreasing waist size, blood lipids, hypertension, blood uric acid levels, hepatic enzymes, and hepatic fat content. Moreover, it advances insulin sensitivity, leading to various metabolic advantages. Presently, crucial Phase 3 research with Mazdutide 4 mg and 6 mg are being conducted among Chinese individuals who are overweight or obese, in addition to patients with type 2 diabetes.