Midazolam Hydrochloride: Detailed Review of its Transformative R&D Success
Midazolam Hydrochloride's R&D Progress
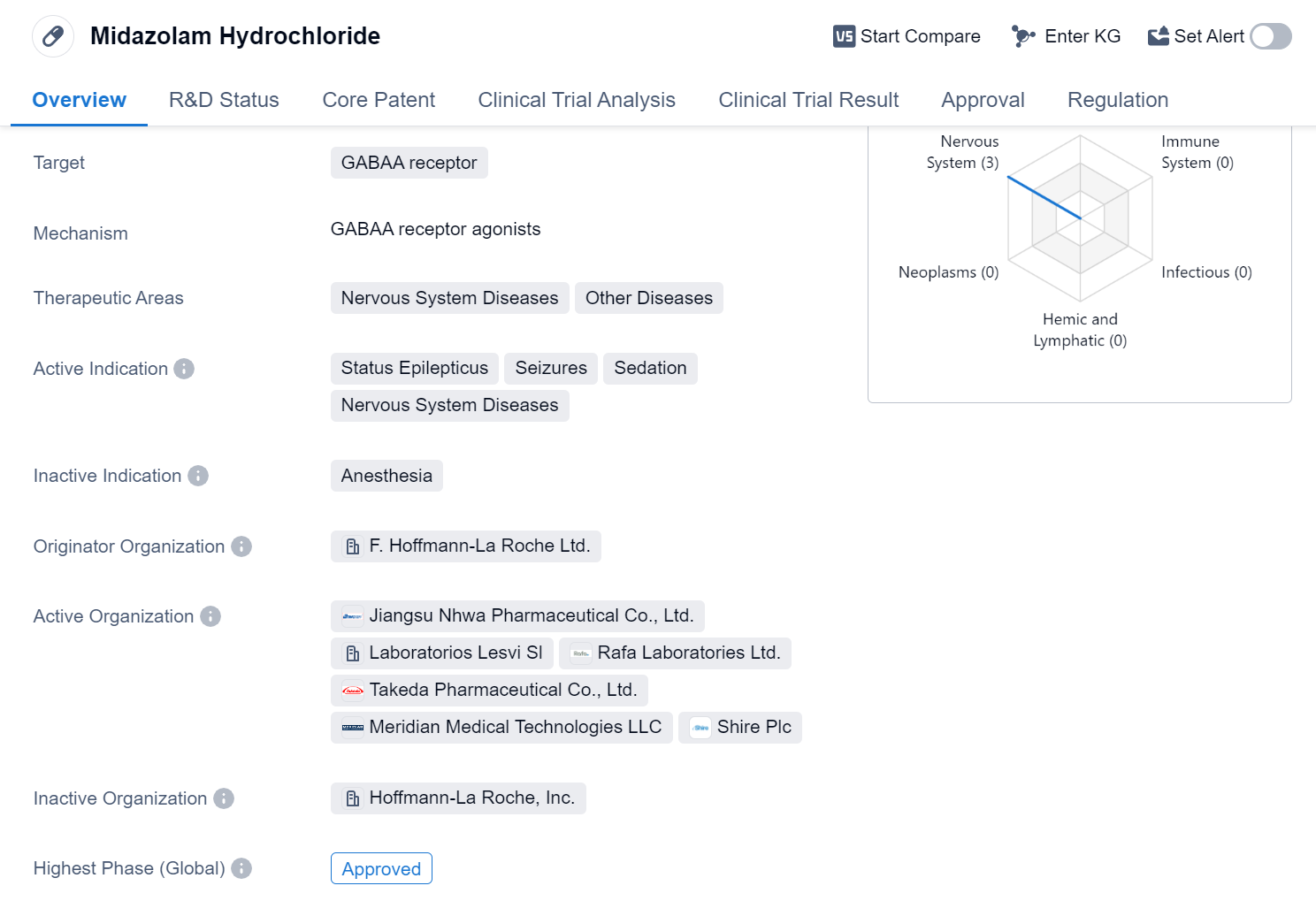
Midazolam Hydrochloride is a small molecule drug that targets the GABAA receptor. It is primarily used in the treatment of nervous system diseases and other diseases. The drug is indicated for the management of status epilepticus, seizures, sedation, and other nervous system diseases.
The originator organization of Midazolam Hydrochloride is F. Hoffmann-La Roche Ltd., a renowned pharmaceutical company. The drug has reached the highest phase of approval globally. It received its first approval in the United States in December 1985, making it a well-established medication in the pharmaceutical market.
Midazolam Hydrochloride is regulated under priority review, which suggests that it has demonstrated significant therapeutic benefits and addresses an unmet medical need. This regulatory status expedites the approval process, allowing the drug to reach patients in a timely manner.
The drug's mechanism of action involves targeting the GABAA receptor, which is a key component of the central nervous system. By binding to this receptor, Midazolam Hydrochloride enhances the inhibitory effects of gamma-aminobutyric acid (GABA), a neurotransmitter that reduces neuronal activity. This leads to sedation and anticonvulsant effects, making it an effective treatment for status epilepticus, seizures, and other related conditions.
Midazolam Hydrochloride has been widely used in clinical practice for several decades. Its approval in the global market highlights its broad therapeutic potential and the recognition of its benefits by regulatory authorities.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Midazolam Hydrochloride: GABAA receptor agonists
GABAA receptor agonists are a type of drugs that bind to and activate the GABAA receptors in the brain. GABAA receptors are a type of neurotransmitter receptor that respond to the neurotransmitter gamma-aminobutyric acid (GABA), which is the primary inhibitory neurotransmitter in the central nervous system.
When GABAA receptor agonists bind to these receptors, they enhance the inhibitory effects of GABA, leading to an overall decrease in neuronal activity. This results in various effects such as sedation, muscle relaxation, anxiolytic (anti-anxiety), and anticonvulsant properties.
These agonists are used in the treatment of various conditions including anxiety disorders, insomnia, epilepsy, and muscle spasms. They can be administered orally, intravenously, or through other routes depending on the specific drug.
It's important to note that GABAA receptor agonists can have sedative effects and may cause drowsiness and impair cognitive and motor functions. Therefore, they should be used with caution and under medical supervision to avoid potential side effects and drug interactions.
Drug Target R&D Trends for Midazolam Hydrochloride
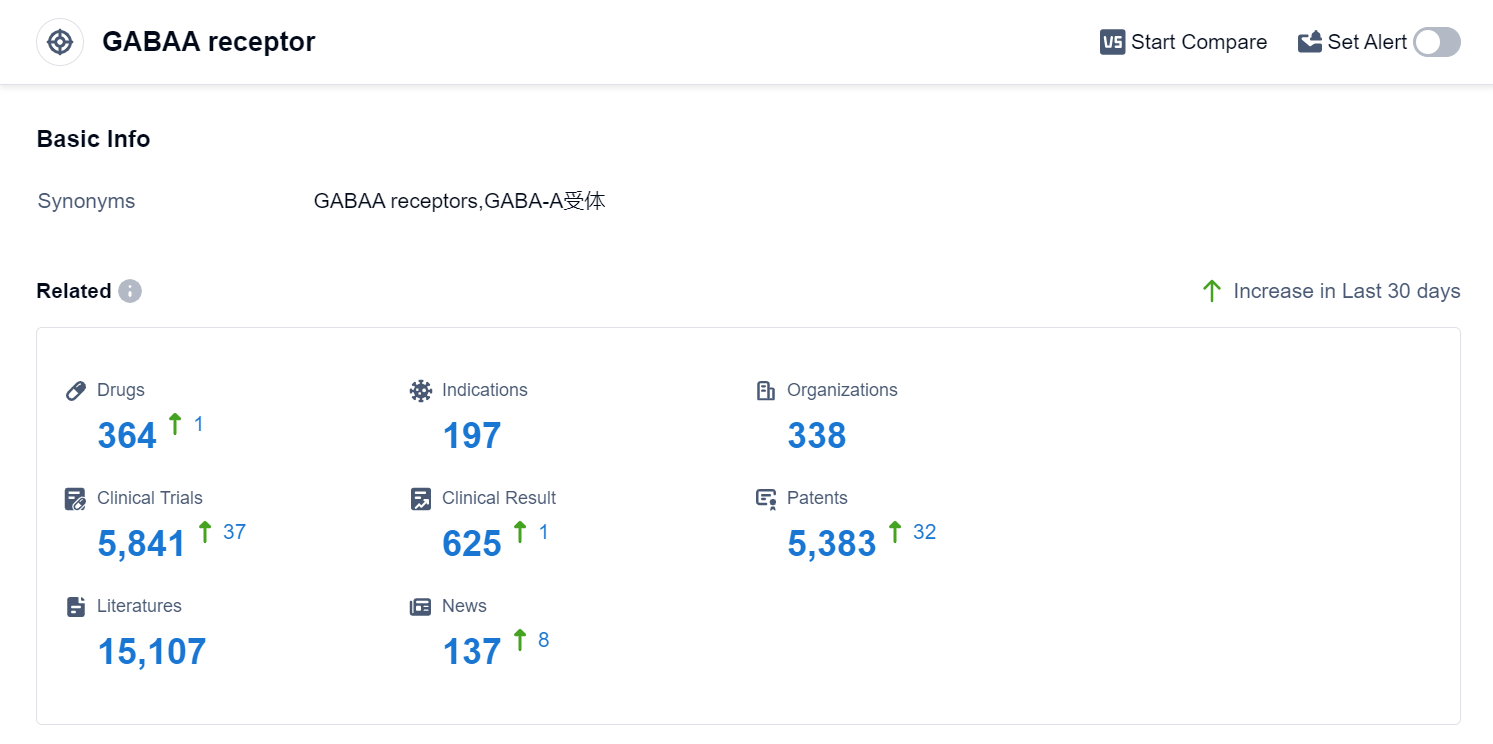
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 364 GABAA receptor drugs worldwide, from 338 organizations, covering 197 indications, and conducting 5841 clinical trials.
The analysis of the target GABAA receptor reveals a competitive landscape with multiple companies actively developing drugs. Pfizer Inc., Sumitomo Chemical Co., Ltd., Teva Pharmaceutical Industries Ltd., Jiangsu Nhwa Pharmaceutical Co., Ltd., and Mitsubishi Chemical Group Corp. are some of the companies with a significant presence in the highest development phases.
Approved drugs for indications such as Sleep Initiation and Maintenance Disorders, Anxiety, Epilepsy, and Seizures indicate a focus on addressing these medical conditions. Small molecule drugs are the primary drug type being developed, with a large number of drugs in the approved and preclinical phases.
Japan, China, and the United States are the leading countries in the development of drugs targeting the GABAA receptor. The progress in China is evident with a significant number of approved drugs and drugs in the preclinical phase.
Overall, the target GABAA receptor presents a competitive landscape with a focus on small molecule drugs and a strong presence in Japan, China, and the United States. Further research and development in these areas can lead to advancements in the treatment of sleep disorders, anxiety, epilepsy, and other related conditions.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Midazolam Hydrochloride is a small molecule drug that targets the GABAA receptor. It is indicated for the treatment of nervous system diseases and other conditions, including status epilepticus, seizures, and sedation. Developed by F. Hoffmann-La Roche Ltd., the drug has received approval in the United States and China. Its priority review status reflects its significant therapeutic benefits, and its mechanism of action involves enhancing the inhibitory effects of GABA. With a long history of use, Midazolam Hydrochloride is a valuable medication in the field of biomedicine.