Pharmaceutical Insights: Menotropins's R&D Progress and its Mechanism of Action on Drug Target
Menotropins's R&D Progress
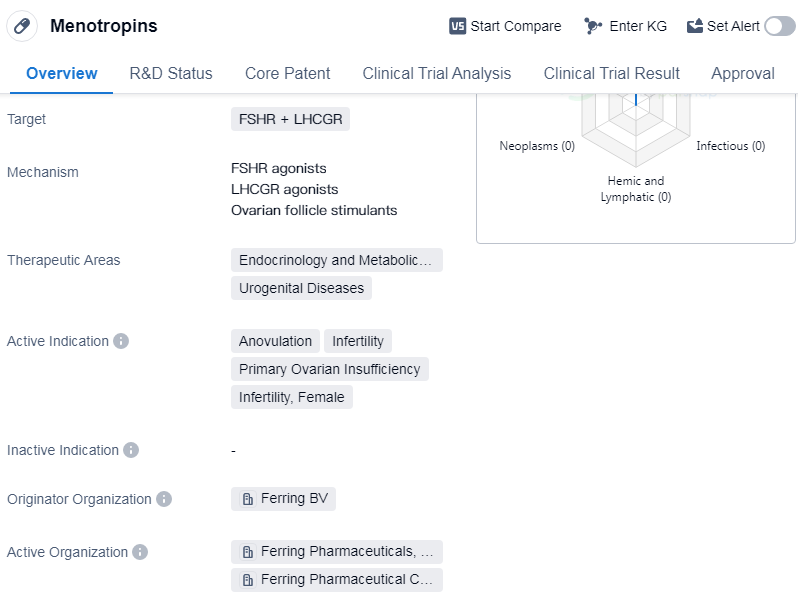
Menotropins is a chemical drug used in the field of biomedicine. It targets the follicle-stimulating hormone receptor (FSHR) and the luteinizing hormone/choriogonadotropin receptor (LHCGR). It is primarily used in the therapeutic areas of endocrinology and metabolic disease, as well as urogenital diseases.
The active indications for Menotropins include anovulation, infertility, and primary ovarian insufficiency in females. It is commonly prescribed to treat female infertility by stimulating the development of ovarian follicles and promoting ovulation. Menotropins can also be used in cases of primary ovarian insufficiency, where the ovaries are unable to produce normal levels of hormones.
Menotropins is classified as a chemical drug, indicating that it is synthesized through chemical processes rather than being derived from natural sources. The originator organization of Menotropins is Ferring BV, a pharmaceutical company specializing in reproductive medicine and women's health.
Menotropins have reached the highest phase of approval globally, indicating that they have undergone extensive clinical trials and have been deemed safe and effective for use. The drug were first approved for use in the United States in August 1999, and it has been approved for use in multiple countries.
In summary, Menotropins is a chemical drug developed by Ferring BV and approved for use in the treatment of anovulation, infertility, and primary ovarian insufficiency in females. It targets the FSHR and LHCGR receptors and is primarily used in the therapeutic areas of endocrinology and metabolic disease, as well as urogenital diseases. Menotropins have been approved globally since 1999 and have achieved the highest phase of approval in the global market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Menotropins: FSHR agonists, LHCGR agonists, and Ovarian follicle stimulants
FSHR agonists, LHCGR agonists, and ovarian follicle stimulants are terms related to biomedicine, specifically in the field of reproductive medicine and fertility treatments.
FSHR agonists refer to follicle-stimulating hormone receptor (FSHR) agonists. FSHR is a receptor found on the surface of ovarian follicles, and when activated by FSHR agonists, it stimulates the growth and development of ovarian follicles. These agonists are commonly used in assisted reproductive technologies, such as in vitro fertilization (IVF), to enhance follicle development and increase the chances of successful ovulation and pregnancy.
LHCGR agonists, on the other hand, refer to luteinizing hormone/choriogonadotropin receptor (LHCGR) agonists. LHCGR is another receptor involved in the reproductive system, specifically in the regulation of ovulation and the production of progesterone. LHCGR agonists stimulate the LHCGR receptor, leading to the release of luteinizing hormone (LH) and triggering ovulation. These agonists are also used in fertility treatments to induce ovulation in women who have irregular or absent ovulation.
Ovarian follicle stimulants encompass a broader category of medications or treatments that aim to stimulate the growth and development of ovarian follicles. This can include both FSHR agonists and LHCGR agonists, as well as other medications like human menopausal gonadotropins (hMG) or recombinant FSH. These stimulants are commonly used in fertility treatments to increase the number of mature eggs available for fertilization, improving the chances of successful conception.
Drug Target R&D Trends for Menotropins
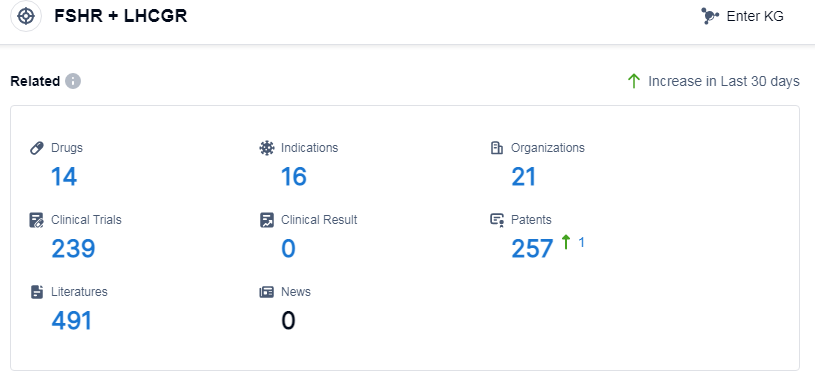
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 14 FSHR and LHCGR drugs worldwide, from 21 organizations, covering 16 indications, and conducting 239 clinical trials.
The analysis of the target FSHR and LHCGR reveals a competitive landscape with multiple companies actively involved in research and development. Merck KGaA, Ferring Holding SA, and Merck & Co., Inc. are some of the companies growing fastest under this target. The indications for drugs targeting FSHR and LHCGR primarily focus on infertility, with other indications also being explored. Hormones and biosimilars progress most rapidly among the drugs, indicating competition in both innovative and biosimilar markets. China, the United States, and the European Union are the countries/locations developing fastest under this target, with China showing significant progress. Overall, the target FSHR and LHCGR presents opportunities for further research and development, particularly in indications beyond infertility and in countries/locations beyond China, the United States, and the European Union.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Menotropins is a chemical drug developed by Ferring BV and approved for use in the treatment of anovulation, infertility, and primary ovarian insufficiency in females. It targets the FSHR and LHCGR receptors and is primarily used in the therapeutic areas of endocrinology and metabolic disease, as well as urogenital diseases. Menotropins have achieved the highest phase of approval in the global market.