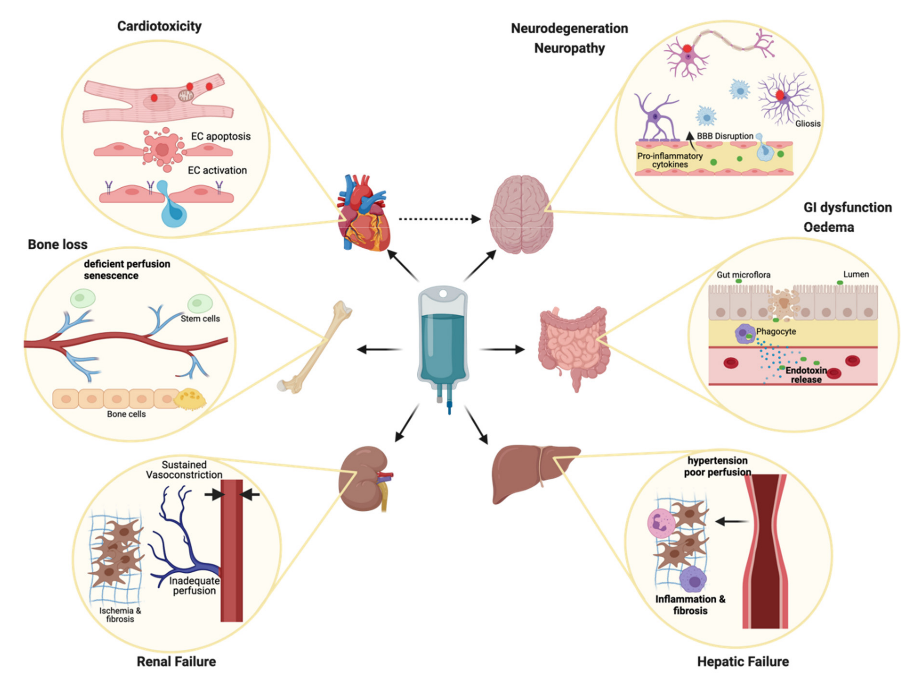
A Sweet Shield Against Chemotherapy's Harsh Side Effects: The Surprising Power of Mannose
Chemotherapy, though a vital and effective treatment in the fight against cancer, is not without its drawbacks. Among these, toxic side effects often significantly diminish patients' quality of life and even impair their ability to withstand and complete their treatment regimen. One of the culprits behind these side effects is a process known as gasdermin-mediated pyroptosis, a form of regulated cell death leading to the release of pro-inflammatory cytokines and damage-associated molecular patterns (DAMPs), which can further amplify tissue damage.
Mannose to the rescue
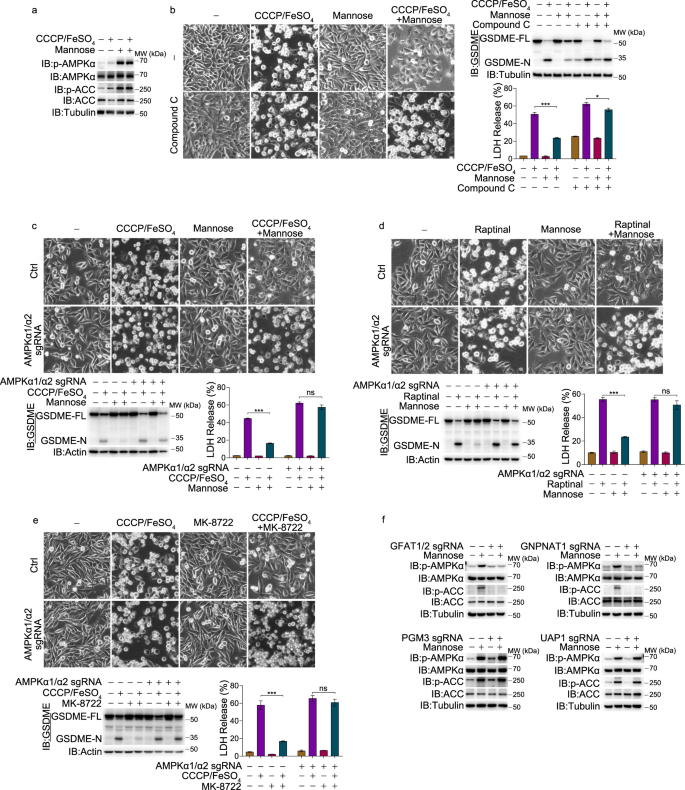
In a quest to alleviate chemotherapy-induced toxicity, a team of researchers from Xiamen University in China embarked on a journey to find a way to inhibit gasdermin-mediated pyroptosis while preserving the antitumor effectiveness of chemotherapy. They uncovered a surprising ally in this fight: mannose, a simple sugar, which proved to be an effective supplement for repressing gasdermin-mediated pyroptosis by activating AMP-activated protein kinase (AMPK). This novel discovery could potentially revolutionize therapeutic strategies for cancer patients undergoing chemotherapy.
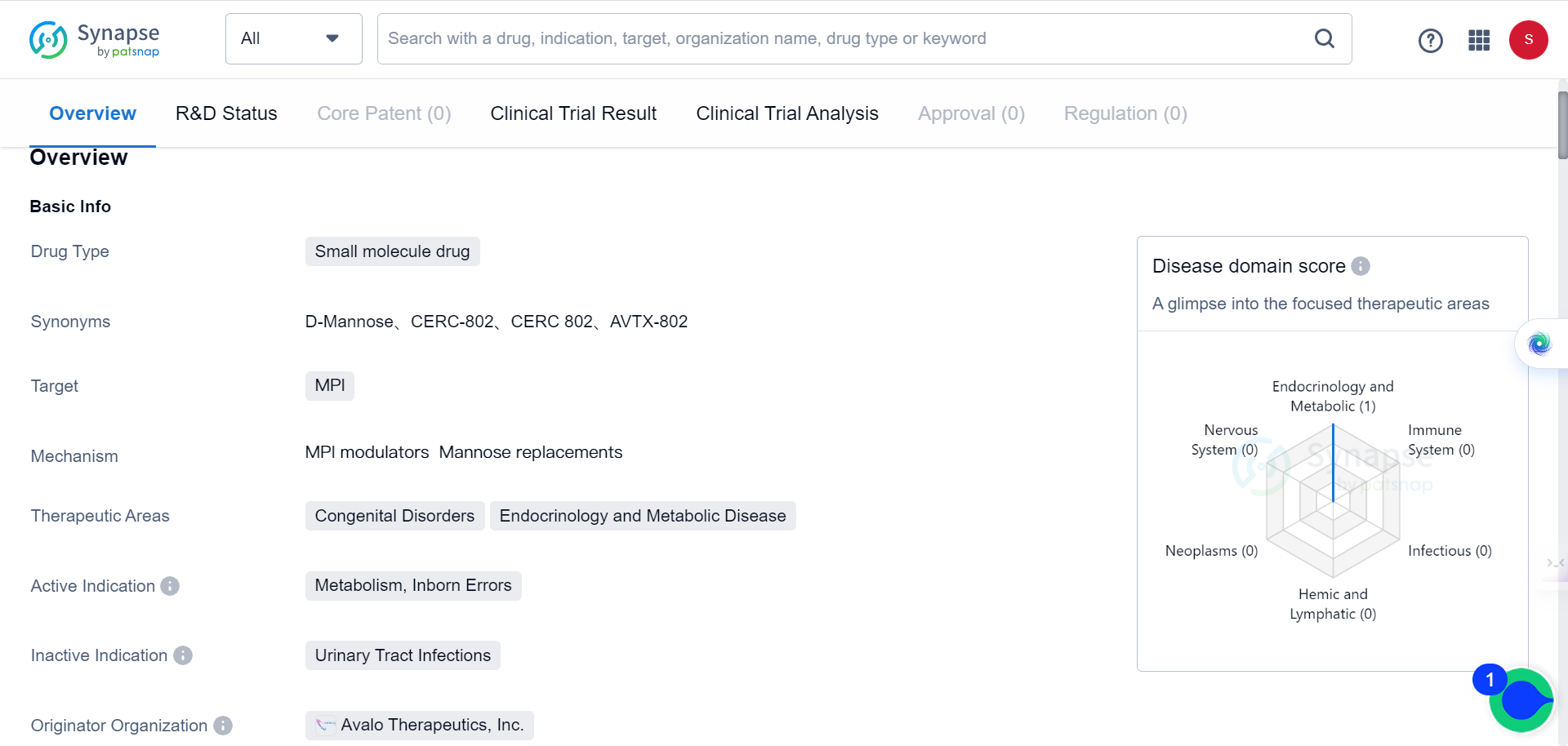
The Synapse database indicates that there is currently only one mannose drug in development by Avalo Therapeutics, Inc.The drug is in the phase 1 trial and is intended for the treatment of congenital disorders as well as endocrinology and metabolic diseases.
The potential use of mannose as a supplement to inhibit gasdermin-mediated pyroptosis by activating AMPK, as described in the study published on Cell Research, presents a novel and promising avenue for the development of cancer treatments, offering a promising approach to mitigating the harsh side effects of chemotherapy.
Minimize the collateral damage
Pyroptosis, the process that instigates cell death, is triggered when gasdermin family proteins cleave and puncture the cell membrane. While this mechanism can prove beneficial in the annihilation of tumor cells, an overzealous activation of pyroptosis in normal tissues can result in damaging side effects, such as nephrotoxicity, lung injury, and gastrointestinal damage.
The study revealed that mannose metabolism in the hexosamine biosynthetic pathway boosts the levels of a metabolite known as N-acetylglucosamine-6-phosphate (GlcNAc-6P). This metabolite binds to AMPK, facilitating its phosphorylation by LKB1. Activated AMPK then phosphorylates GSDME at Thr6, leading to a blockage of caspase-3-induced GSDME cleavage, thereby inhibiting pyroptosis.
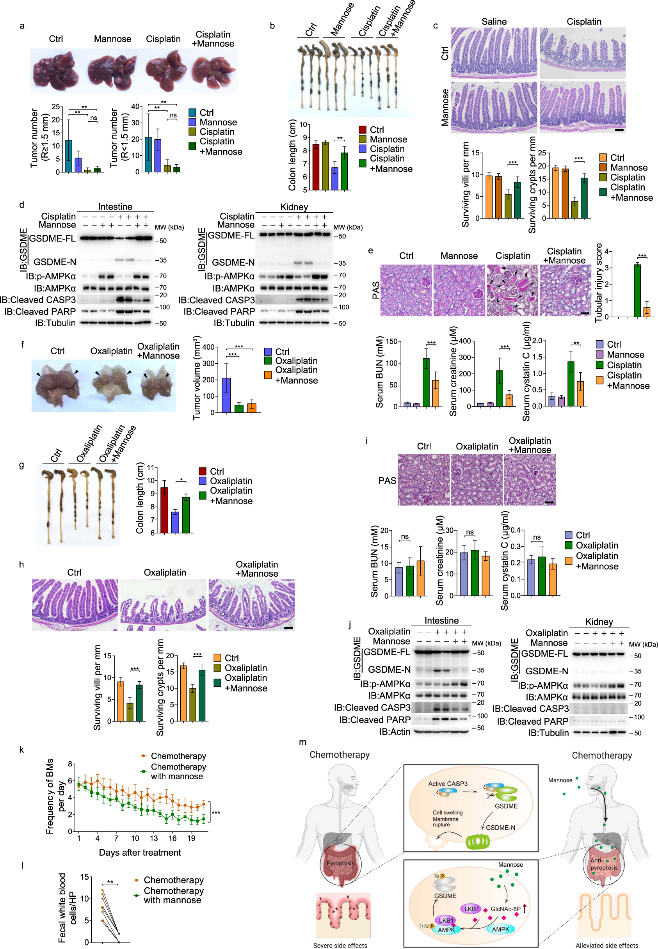
Animal studies on primary cancer models demonstrated that mannose administration could successfully suppress pyroptosis in the small intestine and kidney, thereby alleviating tissue toxicity induced by chemotherapy drugs such as cisplatin or oxaliplatin, without compromising their antitumor effectiveness. The protective power of mannose was further validated in a small cohort of patients with gastrointestinal cancer undergoing chemotherapy, wherein mannose co-administration led to a decrease in diarrhea and fecal white blood cells.
Remarkably, mannose was found not only to be a simple, safe, and selective option for combined treatment to alleviate chemotherapy-induced toxicity, but it also effectively reversed GSDME-dependent pyroptosis induced by cyanide carbonyl and m-chlorophenyl hydrazine (CCCP) plus iron in different melanoma cell lines. No such effect was observed with other hexoses, including glucose, fructose, fucose, and galactose.
AMPK’s role in fighting cancer
This research not only presents a potential alternative therapeutic strategy against melanoma and other cancers, but it also illuminates the regulatory role of activated AMPK in other non-metabolic processes, such as those that dictate cell fate, including autophagy, apoptosis, and ferroptosis.
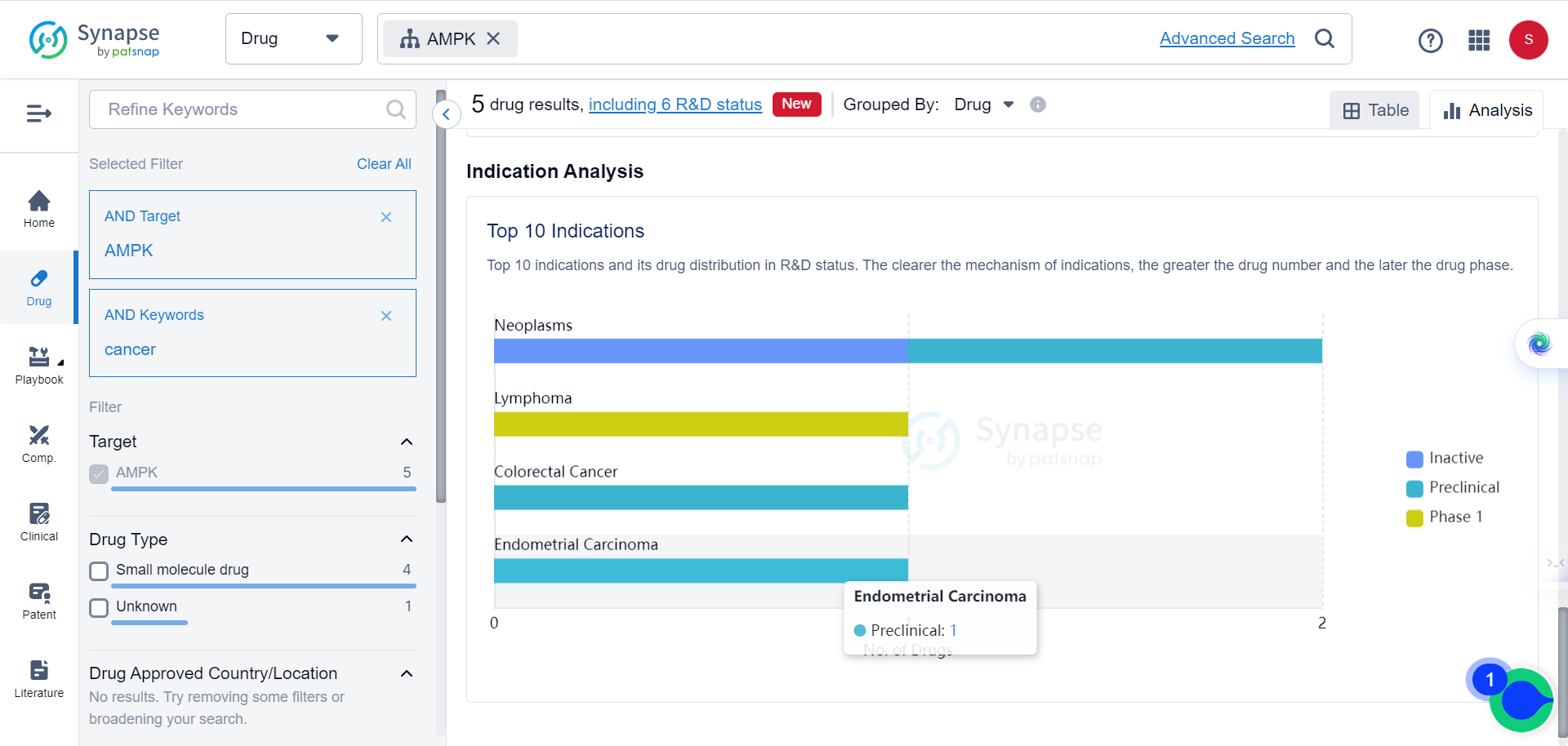
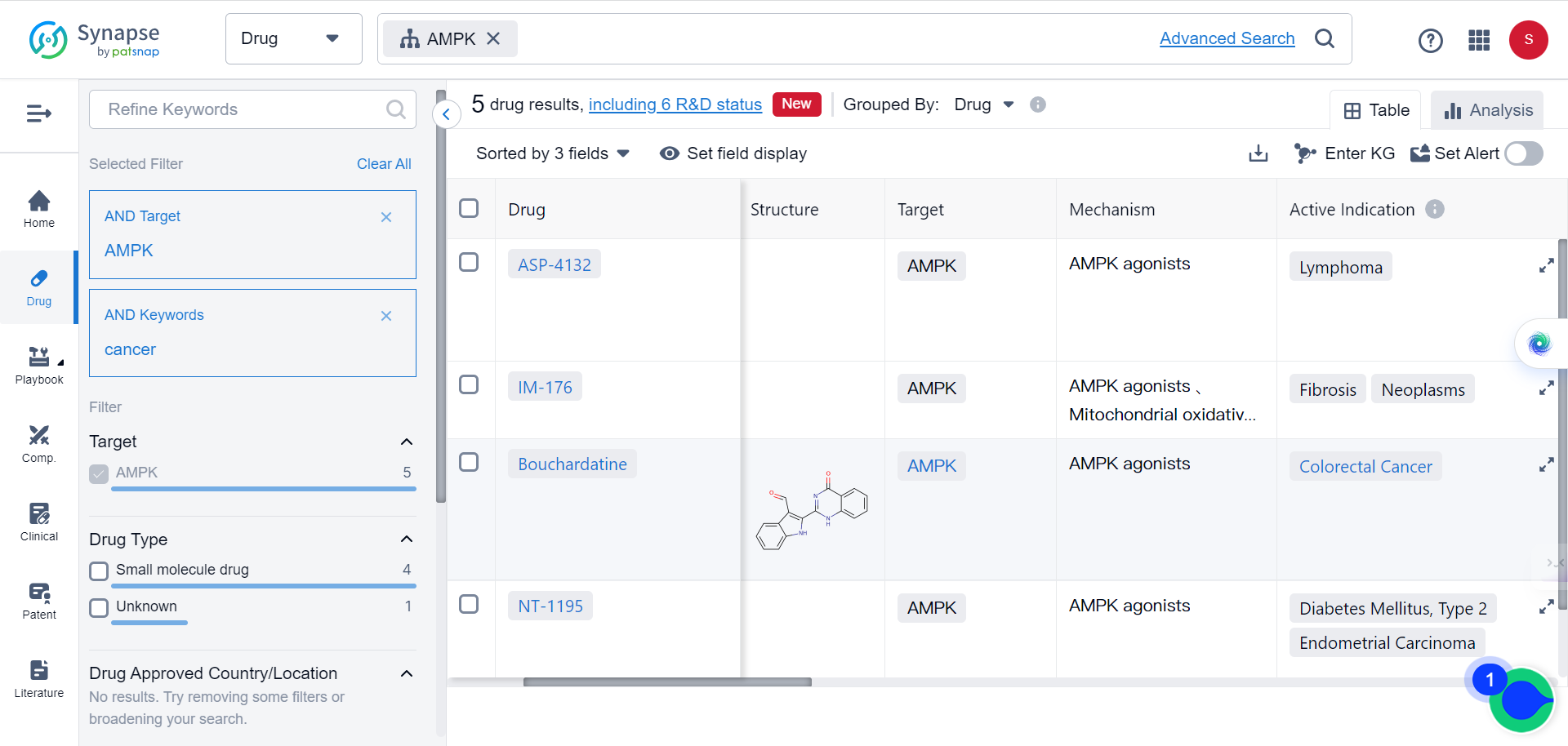
It is noteworthy that according to Synapse data, there are currently five types of AMPK agonists in varying stages of development for cancer treatments.
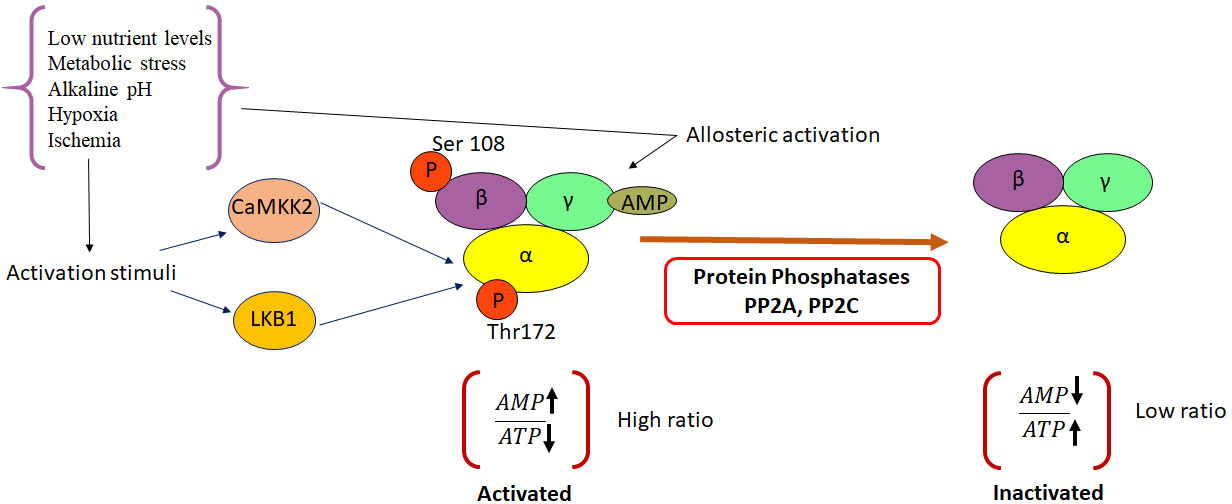
AMPK is a crucial metabolic sensor that plays a key role in maintaining cellular energy homeostasis. Previously, dysregulation of AMPK signaling has been linked to the development of a range of chronic diseases, including obesity, inflammation, diabetes, and cancer.
In cancer, the activation of AMPK and its downstream signaling pathways can lead to dynamic changes in cellular bioenergetics. Studies have illustrated that AMPK acts as an inhibitor during tumor development and progression by modulating both inflammatory and metabolic pathways. Moreover, AMPK functions as a driving factor in the phenotypic and functional transformation of immune cells within the tumor microenvironment (TME).
Numerous studies have highlighted the importance of AMPK in regulating the anticancer effects of various phytochemicals, which are potential candidates for developing anticancer drugs. AMPK-mediated inflammatory responses facilitate the recruitment of specific immune cells to the TME, which in turn can impede the development, progression, and metastasis of cancer.
The novel mechanism whereby mannose antagonizes GSDME-mediated pyroptosis through GlcNAc-6P-mediated activation of AMPK, opening a new frontier of research for developing cancer treatments targeting gasdermin-mediated pyroptosis. These new insights suggest that the sweet power of mannose could be harnessed to shield patients from the bitter side effects of chemotherapy.

References:
1.Wang, S., Wang, H., Feng, C. et al. The regulatory role and therapeutic application of pyroptosis in musculoskeletal diseases. Cell Death Discov. 8, 492 (2022). https://doi.org/10.1038/s41420-022-01282-0
2.Keerthana CK, Rayginia TP, Shifana SC, Anto NP, Kalimuthu K, Isakov N and Anto RJ (2023) The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front. Immunol. 14:1114582. doi: 10.3389/fimmu.2023.1114582