A type of emerging tumor treatment drug: Aptamers
Aptamers are short nucleotide sequences, typically DNA or RNA of 20 to 100 nucleotides. Due to their unique tertiary structure, aptamers can recognize target molecules through their three-dimensional conformation and have a high binding affinity. Compared to antibodies, aptamers have many advantages. Aptamers are products of in vitro chemical synthesis, with the benefits of short synthesis time, low cost, high stability, and strong specificity.
The small and flexible structure of aptamers allows them to bind with smaller targets or hidden structure domains that some antibodies cannot bind to. At the same time, aptamers can also serve as carriers for drug delivery (i.e., Aptamer-Drug Conjugates, ApDC), accurately delivering chemotherapeutic drugs, therapeutic RNAs, toxins and radioactive isotopes into cancer cells.
However, the disadvantages of aptamers are metabolic instability and rapid kidney filtration. Therefore, methods such as chemical modifications and coupling have been developed to overcome these drawbacks, thereby optimizing the development of therapeutic aptamers.
Anti-tumor Therapy of Aptamers
Up to now, most therapeutic aptamers are still in the preclinical or early clinical development stage. Treatment aptamer drugs for ophthalmic diseases, cardiovascular diseases, tumors, and inflammation have entered clinical trials. Pegaptanib, the first nucleic acid aptamer drug for the treatment of wet age-related macular degeneration, was developed by Valeant and approved by the FDA in 2004. It was then successively approved and launched by EMA and PMDA in January 2006 and July 2008, respectively.
Pegaptanib achieves therapeutic effects by binding to vascular endothelial growth factor through spatial structure to inhibit angiogenesis. Subsequently, it faced competition from similar drug Lucentis and its market share declined significantly. Over the past decades, many specific aptamers for various tumor therapies have been developed (Figure 3), for example, breast cancer, colorectal adenocarcinoma, lung cancer, liver cancer, prostate cancer, leukemia, etc.
Currently, there are three types of aptamers undergoing clinical trials for cancer treatment.
The first one is AS1411, which binds to the external structural domain of the target with high affinity. In various tumor models such as lung cancer, breast cancer, kidney cancer, and liver cancer, AS1411 exhibits the ability to inhibit cell proliferation and induce cell apoptosis.
The second one is NOX-A12, an L-type RNA aptamer that is resistant to nuclease degradation. It is an antagonist of chemokine CXCL-12 and can inhibit tumor cell proliferation, angiogenesis, and metastasis.
The third one, AGRO100, can also bind with nucleolin and has been proven to produce inhibitory effects on cell proliferation in various tumor cells. In phase I clinical trials, AGRO100 showed high safety and inhibitory effects on tumor growth. However, up to now, no approved aptamer has been used as a clinical drug for cancer treatment.
Compared to antibodies, the development of therapeutic aptamers has been relatively slow, and there are numerous challenges in developing aptamers into anti-cancer drugs. The slow development is related to the following reasons:
1. Aptamers are oligonucleotides that are easily degraded by nucleases.
2. The small diameter of aptamers enables them to be easily filtered by the kidneys, leading to rapid excretion.
3. As artificially synthesized non-natural nucleotides, aptamers could trigger chemical toxicity or immunogenicity.
4. In a physiological environment, the conformation of chemically synthesized aptamers used ex vivo may change. This change could affect their affinity to the target or their pharmacokinetic characteristics. To overcome the deficiencies of nucleic acid aptamers in terms of affinity, specificity, and stability, various strategies have been adopted to improve the effectiveness of nucleic acid aptamers in the treatment of clinical diseases.
For example, chemical modifications of aptamers, including the introduction of 2'-O-methyl RNA bases, 2'-fluoro, 2'-thiol, and sugar-modified nucleotide analogues. These chemical modification methods have enhanced the resistance of aptamers to nucleases. The binding of aptamers to macromolecules effectively slows down their clearance by the kidneys. These macromolecules include polyethylene glycol, proteins, and inorganic nanoparticles, etc. By utilizing these methods to improve the shortcomings of aptamers, it has greatly promoted the further development of aptamers in disease treatment.
Aptamers, due to their good binding affinity and specificity, have been developed for the treatment of various diseases. They can be used as therapeutics alone, or be covalently/non-covalently bound to drugs as carriers, thus achieving targeted drug delivery. Researchers are currently working on the development of new aptamer drugs for cancer treatment. Although not yet approved, we believe that as a unique, novel anti-cancer drug, they will have a promising future.
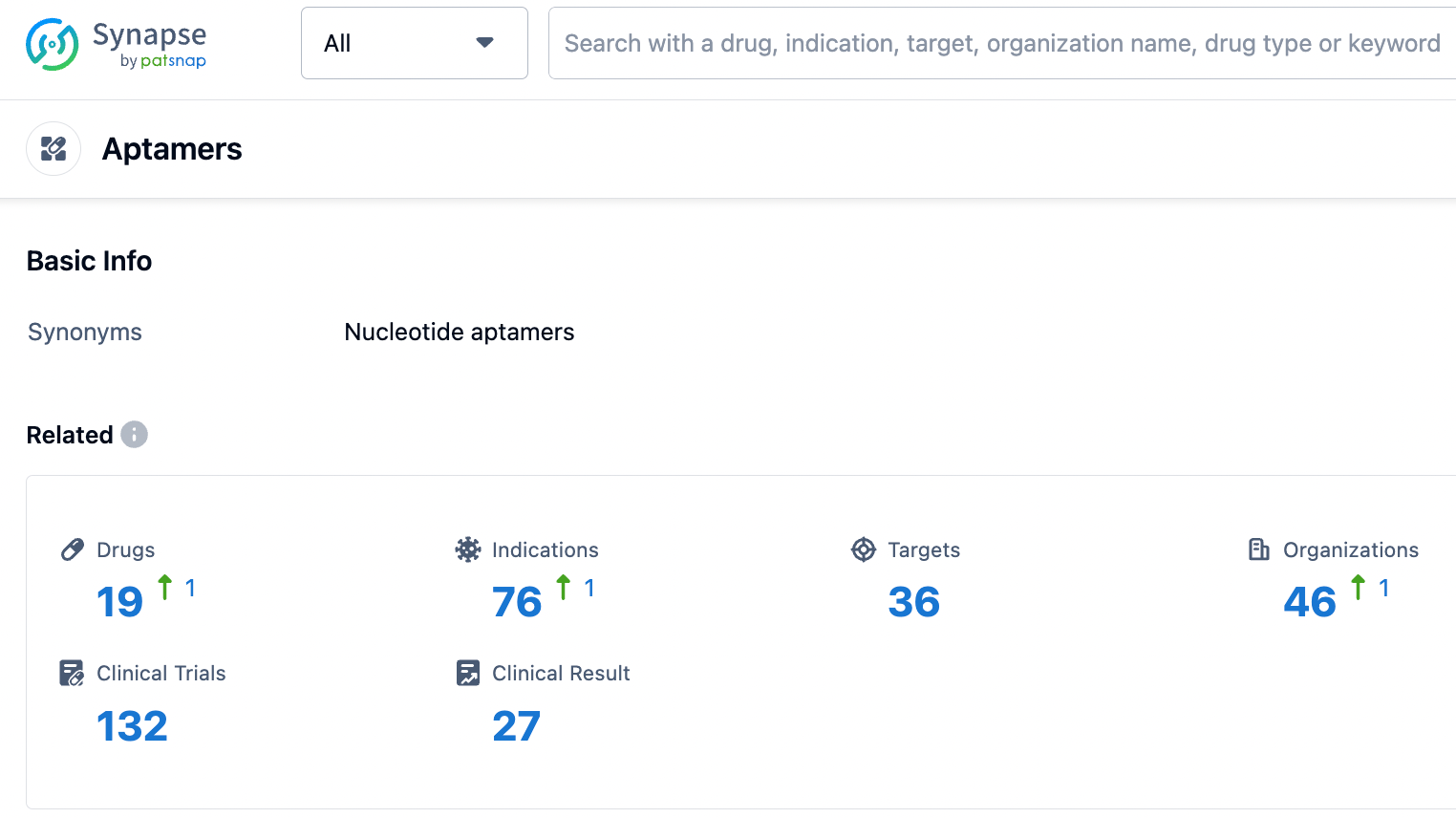
Aptamers Competitive Landscape
According to PatSnap Synapse, as of 19 Sep 2023, there are a total of 19 Aptamers drugs worldwide, from 46 organizations, covering 36 targets, 76 indications, and conducting 132 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this drug type.
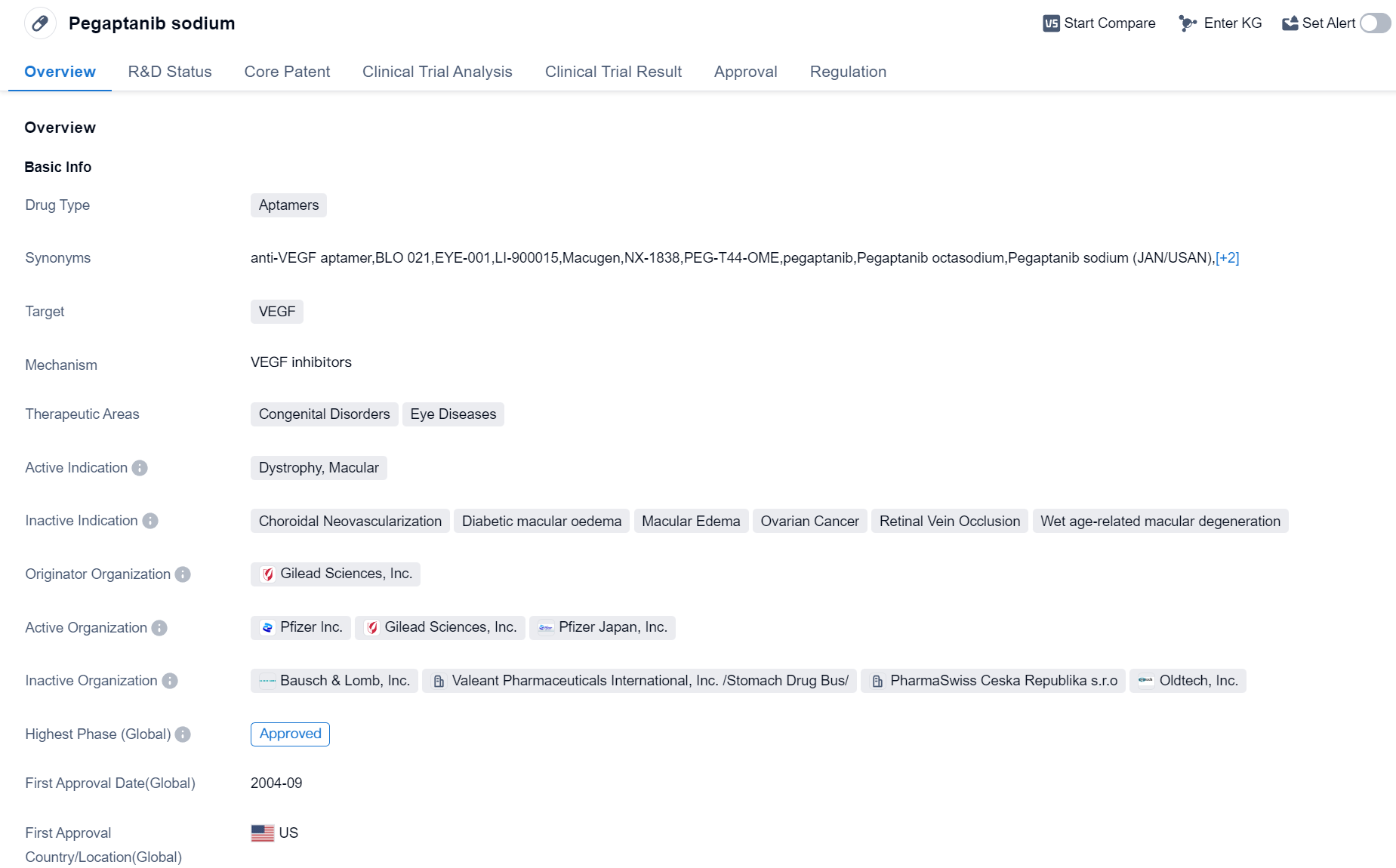
Approved Aptamers Medicinal: Pegaptanib Sodium
Pegaptanib sodium is a drug classified as an aptamer, which means it is a small, single-stranded nucleic acid molecule that binds to a specific target molecule. In the case of pegaptanib sodium, it targets VEGF (vascular endothelial growth factor), a protein that plays a crucial role in the formation of new blood vessels.
The therapeutic areas in which pegaptanib sodium is used are congenital disorders and eye diseases. Specifically, it is indicated for the treatment of dystrophy, macular, a condition that affects the central part of the retina and can lead to severe vision loss.
The drug was developed by Gilead Sciences, Inc., a renowned pharmaceutical company. It received its first approval in the United States in September 2004, making it available for use in patients. The approval was granted under the regulation of orphan drug, which indicates that pegaptanib sodium is intended to treat a rare disease or condition.
As of the highest phase of development, pegaptanib sodium has reached the approved stage globally. This means that it has successfully completed all necessary clinical trials and regulatory requirements to be deemed safe and effective for its intended use.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, pegaptanib sodium is an aptamer drug that targets VEGF and is primarily used in the treatment of dystrophy, macular, a condition affecting the retina. It was developed by Gilead Sciences, Inc. and received its first approval in the United States in 2004. The drug is regulated as an orphan drug, indicating its use in treating a rare disease. Pegaptanib sodium has successfully completed all necessary stages of development and is approved for use globally.