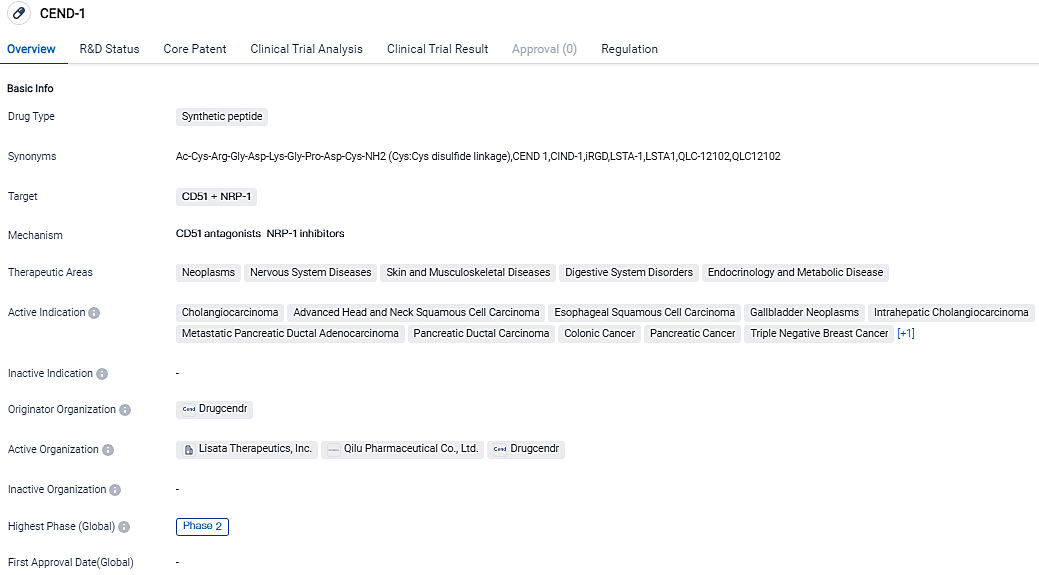
Lisata Therapeutics reports the initial treatment of a patient in the BOLSTER Trial involving LSTA1, for patients with solid tumors
Lisata Therapeutics, Inc., a pharmaceutical firm at clinical-stage that pioneers advanced solutions for treating severe illnesses and complex solid tumours, has revealed the initiation of therapy for the first patient within the head and neck squamous cell carcinoma group as a part of their BOLSTER trial. The treatment was anchored by Dr. Alexander N. Starodub, the research's lead investigator at The Christ Hospital located in Cincinnati, Ohio.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The BOLSTER clinical trial is a Phase 2a, double-blind, placebo-controlled, multi-center, randomized study that examines the efficacy of LSTA1 in conjunction with standard-of-care compared to SOC alone. The patients involved in this study are individuals diagnosed with either advanced second-line head and neck squamous cell carcinoma, second-line esophageal squamous cell carcinoma, or first-line cholangiocarcinoma. Spanning an estimated 40 locations in North America, Europe, and the Asia-Pacific region, the BOLSTER trial is a basket trial.
Total trial enrollment of trial participants is 120, with the anticipated completion in the latter half of 2024. A “basket” trial is one where the same product is tested across multiple separate trial arms, each focusing on different medical conditions with similar traits or challenges, thus providing operational synergies that make the study more efficient than conducting separate trials for each medical condition.
"We are excited to share that the first participant in the second-line head and neck squamous cell carcinoma group of the BOLSTER trial has begun treatment. The BOLSTER trial offers an opportunity for us to assess the capacity of LSTA1 across various solid tumor settings, in combination with matching standard-of-care. This will give us insights into potential next steps in its development." said Kristen K. Buck, M.D., the Executive Vice President of R&D and Chief Medical Officer at Lisata.
“We’ve begun treatment for the first patient in the BOLSTER trial, and we anticipate an increase in trial sign-ups in the next few quarters. We retain our dedication to the development of innovative treatments that could fundamentally change outcomes for patients grappling with such dire health conditions," added Kristen K. Buck.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
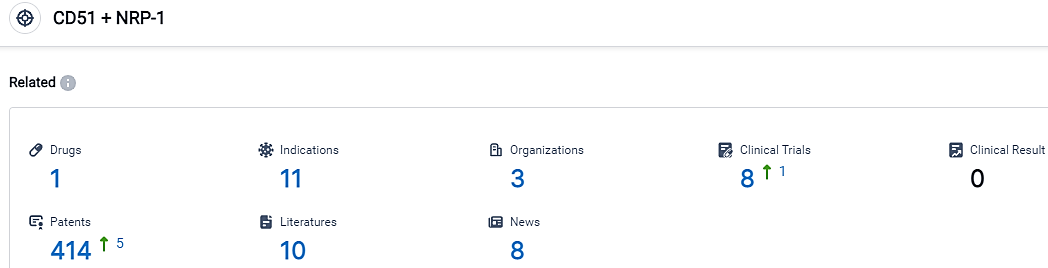
According to the data provided by the Synapse Database, As of September 15, 2023, there are 1 investigational drugs for the CD51 and NRP-1 target, including 11 applicable indications,3 R&D institutions involved, with related clinical trials reaching 8,and as many as 414 patents.
LSTA1 is an investigational drug designed to activate a novel uptake pathway that allows co-administered or tethered anti-cancer drugs to penetrate solid tumors more effectively. LSTA1 actuates this active transport system in a tumor-specific manner, resulting in systemically co-administered anti-cancer drugs more efficiently penetrating and accumulating in the tumor. Lisata is exploring the potential of LSTA1 to enable a variety of treatment modalities to treat a range of solid tumors more effectively.