Regulating tumor immune suppression, the therapeutic effect of the new generation CSF-1R inhibitors is encouraging
Colony-stimulating factor 1 (CSF-1), also known as macrophage colony-stimulating factor, is one of the most common proinflammatory cytokines leading to a variety of inflammatory diseases. It plays a significant role in the development and progression of osteoarthritis, cancer, and other autoimmune diseases. CSF-1 exerts its effects by binding to a receptor called colony stimulating factor 1 receptor (CSF-1R), also known as c-FMS, leading to a signaling pathway cascade, resulting in cell proliferation and differentiation. Although CSF-1R appears to be the only receptor for CSF-1, IL-34 additionally binds to receptor protein-tyrosine phosphatase-zeta (RPTP-ζ) and the expression pattern of the ligand is also different.
CSF-1R/c-FMS is overexpressed in macrophages within many tumors, and thus has been used as a promising target for drug treatment of cancer and inflammatory diseases. Some CSF-1R/c-FMS inhibitors, such as ABT-869, Imatinib, AG013736, JNJ-40346527, PLX3397, DCC-3014, and Ki20227 have been used to study the treatment of these diseases.
CSF-1R inhibitors work by binding to CSF-1R, preventing active cells from the body from signaling, thereby impeding the progression of diseases. In recent years, scientists have explored a series of CSF-1R-targeting drugs based on this target. The research status of related products is mostly at the clinical phase I. Strategies for targeting CSF-1R can be divided into two types. They can use large molecule antibodies to target its CSF-1 ligand, or CSF-1R receptor, or design small molecules to target the kinase active region.
CSF-1R Competitive Landscape
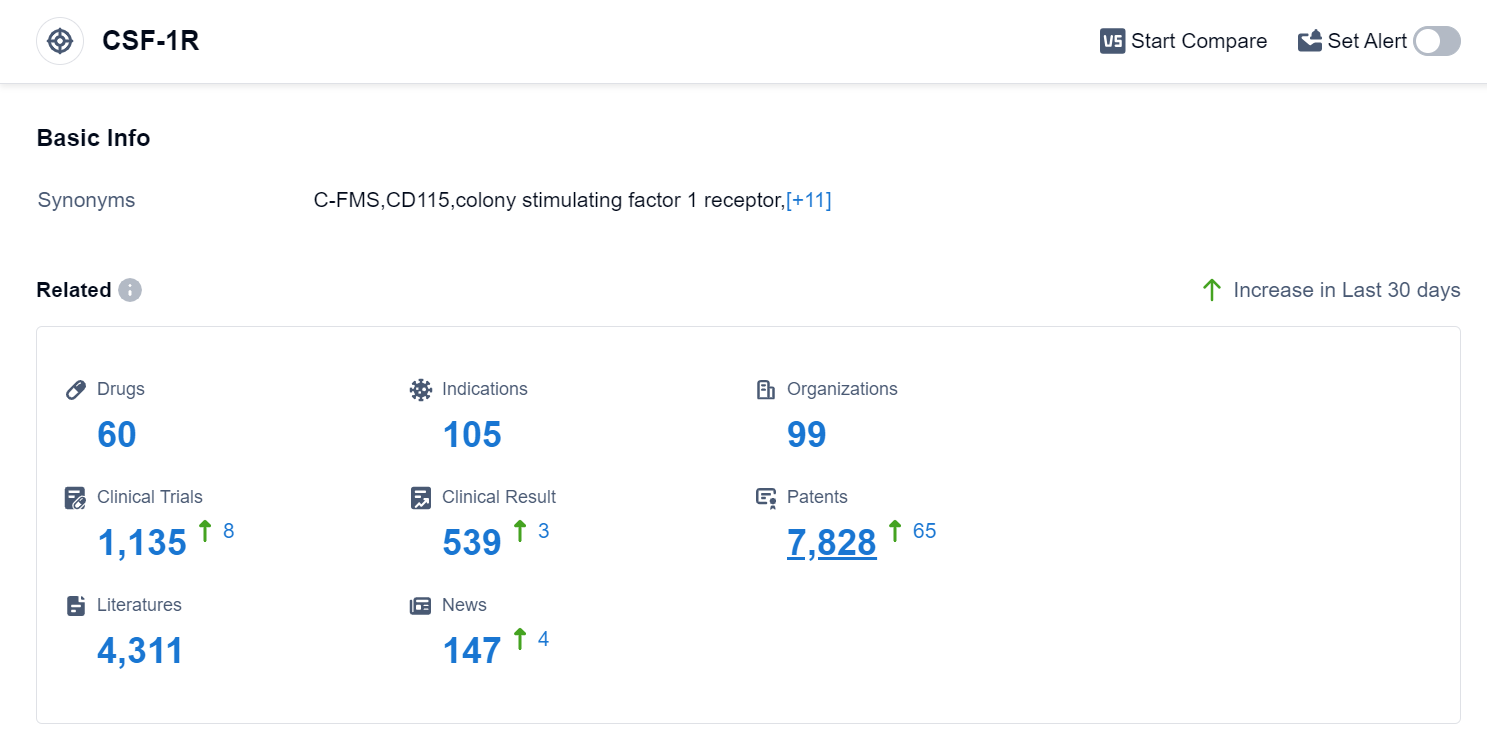
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 15 Sep 2023, there are a total of 60 CSF-1R drugs worldwide, from 99 organizations, covering 105 indications, and conducting 1135 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target CSF-1R reveals a competitive landscape with multiple companies actively involved in the development of drugs. Daiichi Sankyo Co., Ltd., Novartis AG, Hutchison MediPharma Ltd., C.H. Boehringer Sohn AG & Co. KG, Swedish Orphan Biovitrum AB, and JCR Pharmaceuticals Co., Ltd. are some of the companies with significant R&D progress.
The drugs targeting CSF-1R have been approved for various indications, including Giant Cell Tumor of Tendon Sheath, Colorectal Cancer, Hepatocellular Carcinoma, and Non-Small Cell Lung Cancer. Small molecule drugs and monoclonal antibodies are the most rapidly progressing drug types, indicating intense competition.
The United States, China, and Japan are leading in the development of drugs targeting CSF-1R, with China showing significant progress. Overall, the target CSF-1R presents a promising area for future development in the pharmaceutical industry.
Phase III Clinical Trial of CSF-1R Inhibitor: Pimicotinib
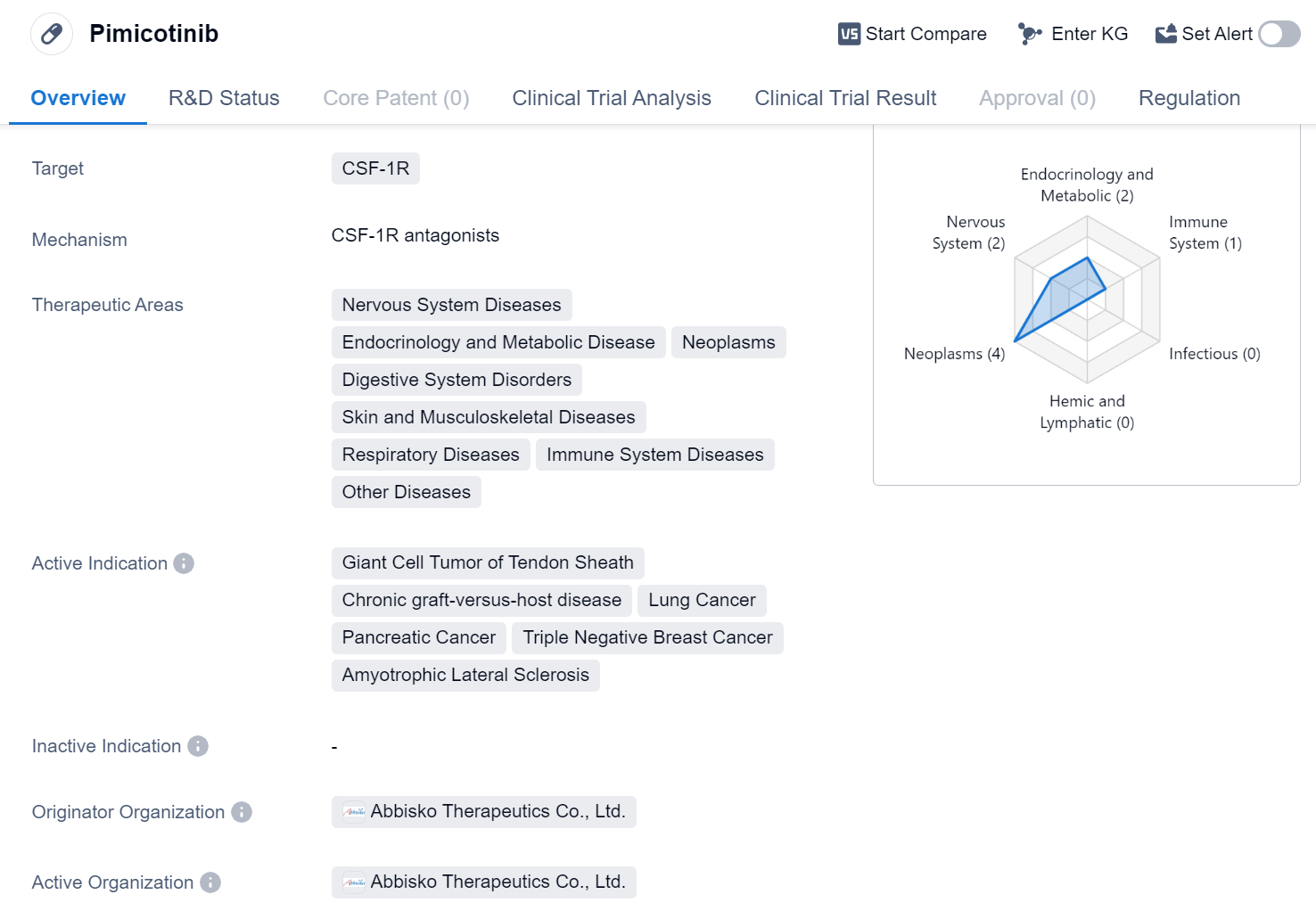
Pimicotinib is a small molecule drug that targets CSF-1R, a receptor involved in various diseases. It has shown potential therapeutic benefits in a wide range of therapeutic areas, including Nervous System Diseases, Endocrinology and Metabolic Disease, Neoplasms, Digestive System Disorders, Skin and Musculoskeletal Diseases, Respiratory Diseases, Immune System Diseases, and Other Diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has been specifically indicated for the treatment of Giant Cell Tumor of Tendon Sheath, Chronic graft-versus-host disease, Lung Cancer, Pancreatic Cancer, Triple Negative Breast Cancer, and Amyotrophic Lateral Sclerosis. These indications cover a diverse range of conditions, highlighting the potential versatility of Pimicotinib in addressing various diseases.
Pimicotinib is developed by Abbisko Therapeutics Co., Ltd., an originator organization in the pharmaceutical industry. The drug has reached the highest phase of clinical development, with Phase 3 trials being conducted globally.
In terms of regulation, Pimicotinib has received PRIME (Priority Medicines) designation, which is a program by the European Medicines Agency (EMA) to accelerate the development of promising medicines for unmet medical needs. Additionally, the drug has been granted Breakthrough Therapy designation, a program by the U.S. Food and Drug Administration (FDA) that expedites the development and review of drugs that demonstrate substantial improvement over existing therapies for serious conditions.
Overall, Pimicotinib is a small molecule drug that targets CSF-1R and has shown potential in various therapeutic areas. With its active indications covering diseases such as Giant Cell Tumor of Tendon Sheath, Chronic graft-versus-host disease, Lung Cancer, Pancreatic Cancer, Triple Negative Breast Cancer, and Amyotrophic Lateral Sclerosis, Pimicotinib holds promise as a versatile treatment option. Its progression to Phase 3 trials globally, along with regulatory designations such as PRIME and Breakthrough Therapy, further highlight its potential as an innovative therapeutic solution in the field of biomedicine.