AbbVie shares findings from its Phase 3 trial involving Venetoclax for individuals with Multiple Myeloma
AbbVie released the results of its Phase 3 CANOVA trial, which assessed the safety and performance of venetoclax (VENCLEXTA®/ VENCLYXTO®) combined with dexamethasone (VenDex) for patients having relapsed or treatment-resistant multiple myeloma who have been administered two or more previous treatments. The data failed to show significant advancement in progression-free survival, the trial's main objective.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
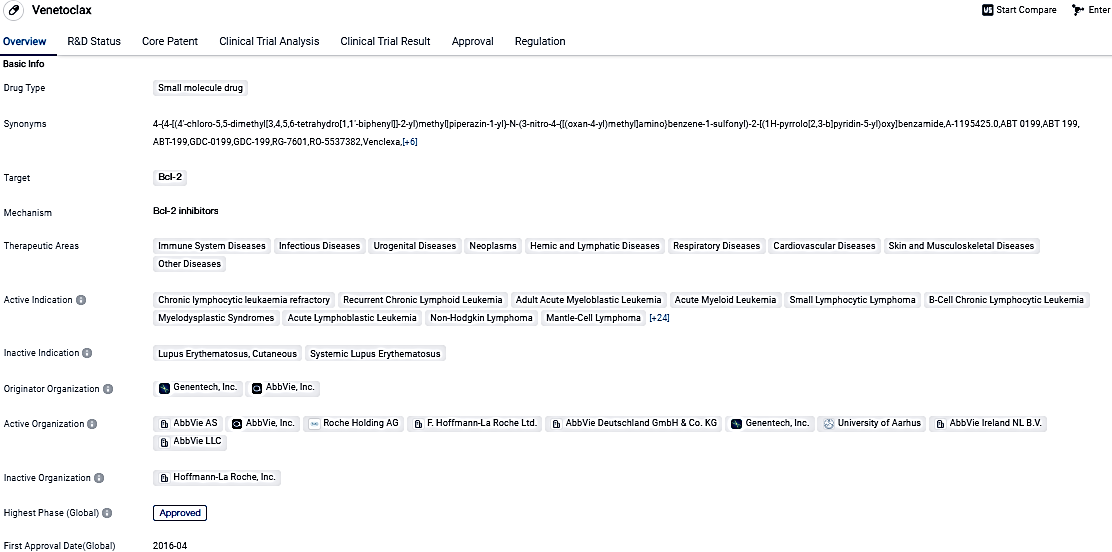
Venetoclax, a small molecule drug that targets Bcl-2, received approval for treating numerous conditions spanning various therapeutic fields. Gaining approval in the United States in 2016, the drug emerged as a significant breakthrough in the arena of biomedicine. Its potential to manage unaddressed medical necessities in patients with an array of diseases is underscored by its effectiveness and regulatory designations.
The combination of venetoclax and dexamethasone remained generally safe throughout the trial, maintaining consistency with the known safety profiles of the individual agents. No new safety issues arose. Patients treated with VenDex predominantly encountered side effects such as infections, diarrhea, lymphopenia, and nausea. Patients treated with PomDex often faced adverse effects like neutropenia, infections, thrombocytopenia, and anemia.
Multiple myeloma ranks as the world's second most prevalent blood cancer. Most patients suffer poor prognoses as the majority relapse even with recent advancements in treatment advances. A certain group of patients carry the biomarker, a type of chromosomal translocation frequently seen in multiple myeloma, that may lead to an overproduction of the BCL-2 protein.
Mariana Cota Stirner, M.D., Ph.D., therapeutic area head oncology hematology at AbbVie stated, "Although the CANOVA trial didn't achieve its main goal, the favorable tendencies observed throughout the study prompt us to consult these results with health officials soon. We continue to be resolute in our commitment to boost the standard of care for blood cancer sufferers globally, encompassing those with multiple myeloma."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
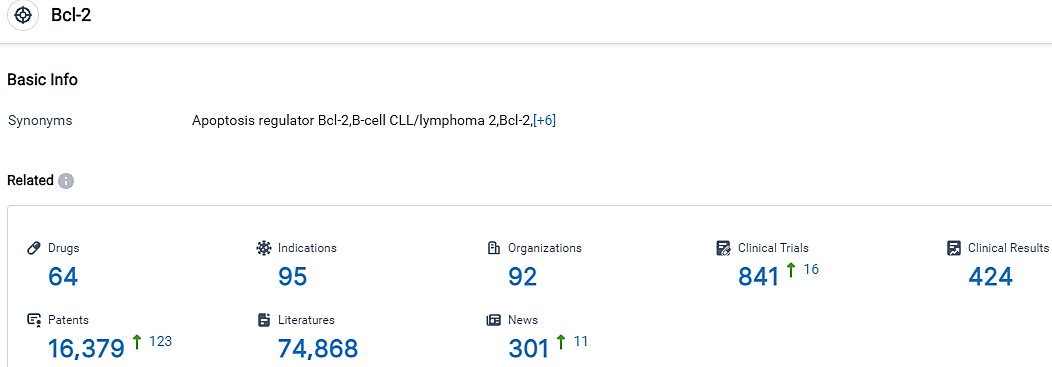
According to the data provided by the Synapse Database, As of October 9, 2023, there are 64 investigational drugs for the Bcl-2 target, including 95 indications, 92 R&D institutions involved, with related clinical trials reaching 841,and as many as 16379 patents.
VENCLEXTA/VENCLYXTO (venetoclax) is a first-in-class medicine that selectively binds and inhibits the B-cell lymphoma-2 (BCL-2) protein. Venetoclax is currently approved for patients with previously untreated and treated chronic lymphocytic leukemia and newly diagnosed acute myeloid leukemia.