AEON Biopharma Discloses Encouraging Outcomes of ABP-450 Phase 2 Clinical Study in Cervical Dystonia
AEON Biopharma, Inc., a company in the clinical-stage biopharmaceutical field working specifically on creating a unique botulinum toxin complex as a remedy for numerous incapacitating health afflictions, has shared encouraging results from their recent Phase 2 medical trial of ABP-450. This potential treatment, aimed at cervical dystonia - a persistent and debilitating condition that influences neck muscles, was presented at the International Parkinson and Movement Disorders Society Congress®. The congress took place at the Bella Center, Copenhagen, Denmark, from August 27 to 31, 2023. The company had initially made this data public in September 2022.
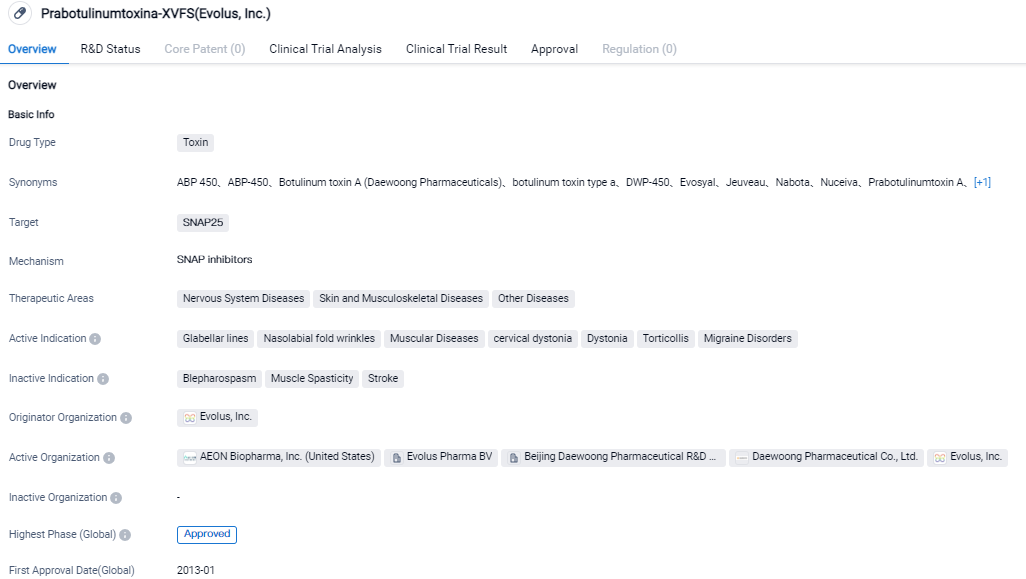
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The display panel captioned, "A Stage 2, Double-blinded, Placebo-supervised Test to Assess the Efficiency and Safety of ABP-450 (PrabotulinumtoxinA) in Adults Experiencing Singular Cervical Dystonia," was disclosed by Chad Oh, Chief Medical Officer of the organization. The display reveals full details from the Stage 2 probe, proving that the twin lesser doses (150 units and 250 units) of ABP-450 brought about statistically noteworthy enhancements in Toronto Western Spasmodic Torticollis rating scale (TWSTRS) cumulative scores from the starting point to Week 4, while the superior dose (350 units) showed numerical betterment compared to placebo. The entire doses of ABP-450 within the probe demonstrated sustained advantages, with the median period of effect over all dose groups being at least 20 weeks, the patients' final appointments. At Week 4, augmentation in Clinical Global Impression of Change and Patient Global Impression of Change ranks held statistical worthiness in each dose of ABP-450 versus the placebo. ABP-450 was generally secure and patients with CD tolerated it well.
"AEON's Phase 2 data shown at IP-MDS is crucial to this scheme as the TWSTRS marks and safety stats reinforce our strategy to go after ABP-450 as an authenticated therapy for CD," stated Marc Forth, AEON's CEO and President. "We're eager to move forward with ABP-450's evolution as a treatment alternative for those suffering from this incapacitating predicament. Presently, we are conversing with the FDA about the arrangement of a Stage 3 probe in CD, which we intend to kick off in 2024, contingent on the accessibility of capital assets. Plus, we're consistently on course to voice the major data from our currently running Stage 2 probe of ABP-450 effecting episodic migraine in the fall of 2023."
On the subject of AEON's Stage 2 Cervical Dystonia Test
The Stage 2 test (ClinicalTrials.gov Identifier: NCT04849988) is a randomized, double-blind, placebo-supervised study that evaluated 57 patients total, spread across 20 examination locations in the United States. They apportioned patients evenly amongst four groups, which included a low dosage (150 units), mid-size dosage (250 units), and high dosage (350 units) treatment of ABP-450, as well as a placebo. They gave every patient a sole treatment cycle of their selected dose of ABP-450 or placebo. Patients were tracked until the 20th week, and evaluated the primary efficacy endpoint at four weeks after dosage. Due to the nature of the disease, dosing is individualized to the patient by the investigator based on the severity of the patient’s head and neck position, pain location, muscle bulging, patient response, and adverse event history.
Following the conclusion of the Stage 2 clinical trial, all patients, regardless of their treatment group, had the choice to receive ABP-450 treatment by transitioning into a 52-week open-label extension study, and 51 out of the 57 patients (89%) chose to proceed.
ABP-450 comprises a 900 kDa Clostridium botulinum bacterium-produced botulinum toxin type-A complex. The toxin's active part is its 150 kDa component, and the leftover 750 kDa complex consists of accessory proteins that the Company thinks assist with the active botulinum toxin's function. When injected at therapeutic levels, ABP-450 prevents peripheral acetylcholine release at presynaptic cholinergic nerve terminals by cleaving SNAP-25, a protein necessary to the successful docking and release of acetylcholine from vesicles situated within the nerve endings leading to denervation and relaxation of the muscle.
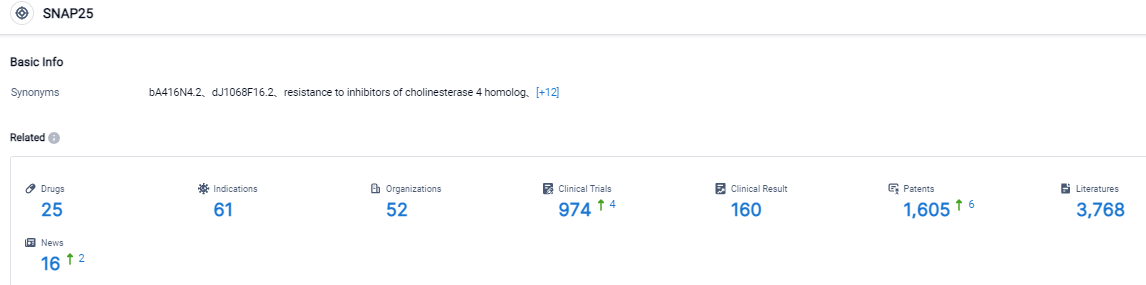
Click on the image below for direct access to the latest R&D progress on SNAP-25, target drugs, indications, research institutions, clinical trials, and more. As of August 31, 2023, there are 25 investigational drugs for the SNAP-25, target, including 61 applicable indications, 52 R&D institutions involved, with related clinical trials reaching 974 and as many as 1605 patents. ABP-450, a botulinum toxin complex, is presently authorized and promoted by Evolus for aesthetic applications and is known as Jeuveau. Daewoong manufactures ABP-450, adhering to current Good Manufacturing Practice standards in a facility approved by the FDA, Health Canada, and the European Medicines Agency. AEON holds the unique developmental and distribution authorization for ABP-450's therapeutic uses in the US, Canada, the EU, the UK, and several other global areas. The firm has assembled a management team, abundant with experience in the biopharmaceutical field and botulinum toxin development and marketing.