The European Commission Approves KEYTRUDA® (pembrolizumab) plus Trastuzumab and Chemotherapy, as a treatment for HER2-Positive Advanced Gastric or Gastroesophageal Junction
Merck announced that Merck announced that the European Commission has approved KEYTRUDA, Merck’s anti-PD-1 therapy, in combination with trastuzumab, fluoropyrimidine- and platinum-containing chemotherapy, for the first-line treatment of locally advanced unresectable or metastatic human epidermal growth factor receptor 2 (HER2)-positive gastric or gastroesophageal junction adenocarcinoma in adults whose tumors express PD-L1 (Combined Positive Score≥1).
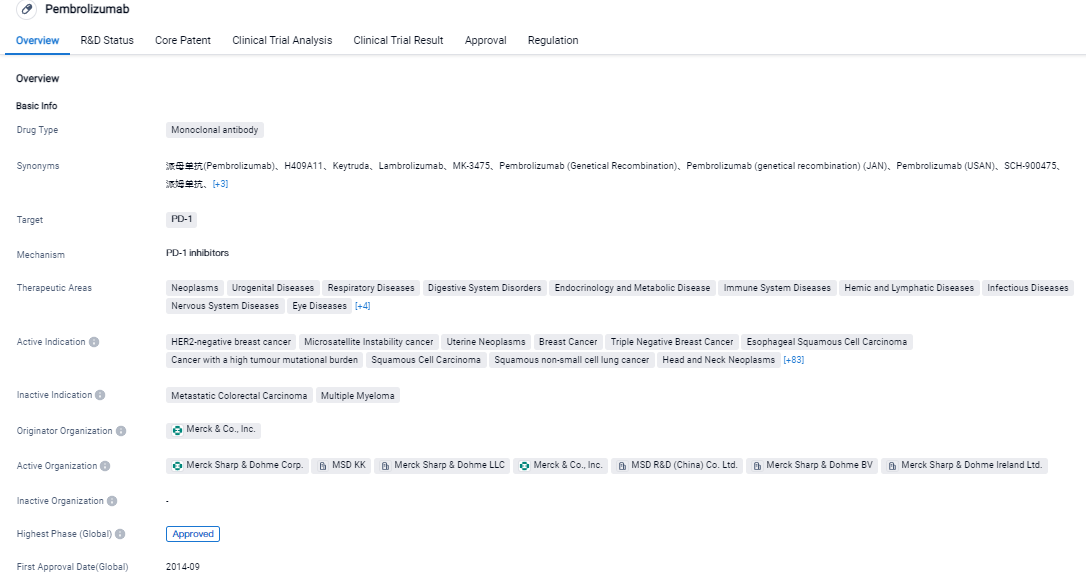
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The study revealed that when used in conjunction with trastuzumab and chemotherapy, KEYTRUDA greatly increased progression-free survival and objective response rate, as opposed to trastuzumab and chemotherapy alone in the same patient cohort. Moreover, over 80% of the patients had tumors that were positive for PD-L1.
While there was an increase in overall survival, another primary endpoint of the trial for patients in the ITT group who got the KEYTRUDA combination as opposed to placebo alongside trastuzumab and chemotherapy, these results didn't reach statistical significance in accordance with the pre-established statistical analysis strategy. The OS evaluation for this trial is still being conducted.
Upcoming 2023 European Society for Medical Oncology Annual Meeting will present the findings from KEYNOTE-811.
Dr. Scot Ebbinghaus, VP at Merck Research Laboratories, stated that patients diagnosed with HER2-positive advanced gastric cancer in the EU have a formidable disease with dismal prognosis. Thus, there is an increased need for more initial treatment options. KEYTRUDA's approval today brings hope to the patients with a combined positive score of ≥1 PD-L1 tumor expression and healthcare providers in the EU to include immunotherapy as a treatment option for this challenging disease.
Following this approval, KEYTRUDA regimen can now be marketed in all the 27 EU member nations as well as Iceland, Lichtenstein, Norway, and Northern Ireland.
In May 2021, KEYTRUDA, in combination with trastuzumab, fluoropyrimidine- and platinum-based chemotherapy received approval for the initial treatment of locally advanced irreparable or metastatic HER2-positive gastric or GEJ adenocarcinoma patients in the U.S. FDA gave accelerated approval for this indication based on the ORR data from KEYNOTE-811. Full approval is dependent on verification and explanation of clinical benefits in the follow-up trials. Merck is collaborating with FDA to widen this indication to patients whose tumors are PD-L1 positive.
Merck is investing in its broad-scale clinical development program to explore KEYTRUDA's potential role in gastrointestinal cancers. They are studying its applications across various cancers such as gastric, hepatobiliary, esophageal, and colorectal.
About KEYNOTE-811:
KEYNOTE-811, a random double-blind Phase 3 study is evaluating KEYTRUDA in combination with trastuzumab and chemotherapy for initial treatment of locally advanced irreparable or metastatic HER2-positive gastric or GEJ adenocarcinoma. PFS as assessed by blinded independent central review and OS are the dual primary endpoints. The secondary endpoints include ORR, response duration, and safety.
About KEYTRUDA®(pembrolizumab) injection, 100 mg:
KEYTRUDA is an anti-PD-1 therapy, aimed to increase the body’s immune system’s capability to detect and fight tumor cells. It is a humanized monoclonal antibody that interrupts the interaction between PD-1 and its ligands, PD- L1 and PD-L2, activating T lymphocytes that may affect both tumor and healthy cells.Merck boasts the largest immuno-oncology clinical research program across the industry. Presently, over 1,600 KEYTRUDA trials are ongoing across various cancers and treatment methods. The aim of the KEYTRUDA clinical program is to comprehend its role across various cancers and factors that might predict a patient’s chances of benefiting from KEYTRUDA treatment. This includes the exploration of a variety of biomarkers.
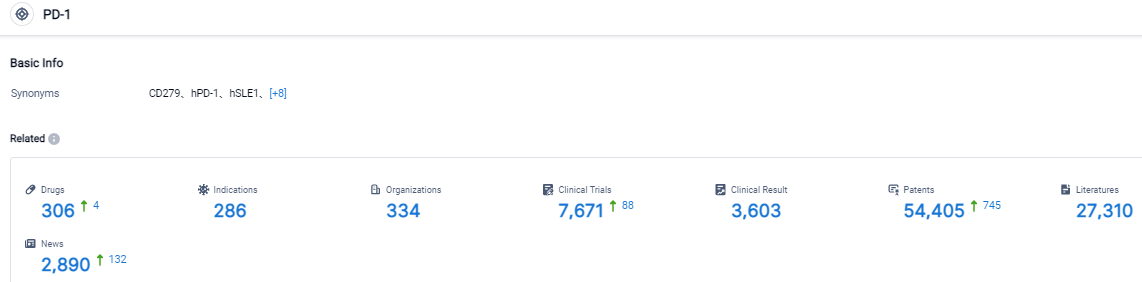
Click on the image below for direct access to the latest R&D progress on PD-1 target drugs, indications, research institutions, clinical trials, and more. As of August 31, 2023, there are 306 investigational drugs for the PD-1 target, including 286 applicable indications, 334 R&D institutions involved, with related clinical trials reaching 7671, and as many as 54405 patents. Keytruda (pembrolizumab) is a humanized monoclonal antibody designed to inhibit the PD-1 co-inhibitory receptor. The interaction between PD-1 and its primary ligand, PD-L1, inhibits T-cell proliferation and survival, and induces apoptosis of tumor-specific T-cells. The disruption of this interaction reduces the ability of the tumor to evade immune system targeting and enhances antitumor immune responses. It has also been hypothesized that blocking the PD-1/PD-L1 interaction may preferentially release the cytotoxic function of tumor-specific T cells with fewer toxic effects than those seen with other immune checkpoint inhibitors.