TScan Therapeutics announces their IND application for TSC-203-A0201 received clearance from the FDA
TScan Therapeutics, Inc., a biopharmaceutical firm in the clinical-stage focusing on the creation of T cell receptor engineered T cell medical solutions for cancer patients announced today that the U.S. Food and Drug Administration has given approval for its new experimental drug TSC-203-A0201, a TCR-T pinpointing PReferentially expressed Antigen in Melanoma. Besides its expression in approximately 90% of melanomas, PRAME is also commonly expressed in various different types of solid tumors such as an approximate 90% of head and neck cancers and around 50% of non-small cell lung cancers. TSC-203-A0201 is designated for patients with a specific HLA type A*02:01 which accounts for more than 40% of the population in U.S. TSC-203-A0201 is the fourth TCR-T approved for clinical advancement in the company's solid tumor program, coming after TSC-200-A0201, TSC-204-A0201, and TSC-204-C0702, which aim at human papillomavirus 16 (HPV16) displayed on HLA type A*02:01, and MAGE-A1 displayed on HLA categories A*02:01 and C*07:02, respectively.
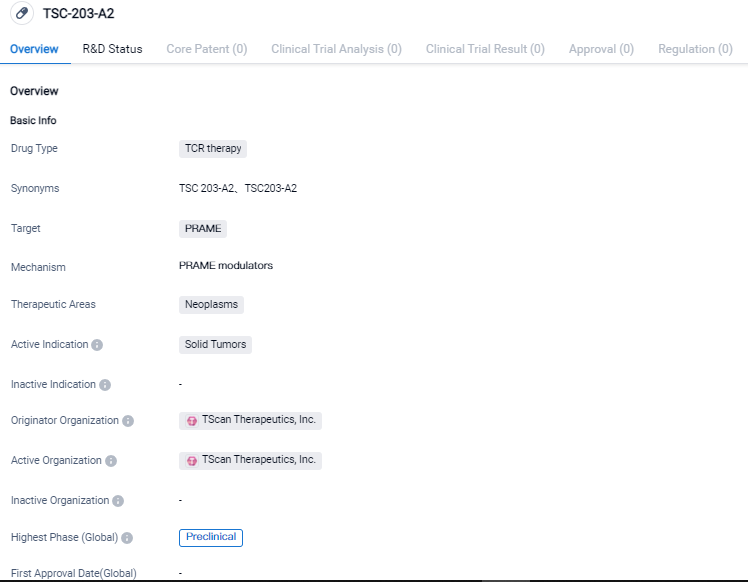
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Each of the four TCR-T cell products is engineered to be interoperable with others under a uniform clinical trial protocol. Each product is an improved, self-replicating T cell treatment embedded with a tumor antigen-specific TCR, accompanied by CD8α/ß to stimulate helper T cells and a prevailing negative version of TGFß receptor II to boost T-cell longevity. TCR-T cell treatments are tailored to patients based on the specific HLAs and targets their tumors express. In the initial clinical trial stage, every agent will be evaluated individually at two dosage levels for safety before being approved for amalgamation with any other TCR-T cell product.
"We're very excited to maneuver a strategy that lets us tweak treatments according to each patient's tumor biology, especially for prevalent cancers like melanoma, lung, and head and neck," commented Dr. Debora Barton, Chief Medical Officer. "On a higher note, adjustable treatments could be imperative to sustaining responses, confronting tumor diversity, and dealing with resistance due to HLA or target loss, which are usually seen in these tumor types. We've kicked off a screening protocol to find suitable patients, and have started preparing for the clinical trial of all four TCR-Ts in the ImmunoBank. We are enthusiastic about the chance to help patients suffering from these common solid tumors and are proceeding as planned to treat the first patient with a TScan TCR-T and provide early data by the end of 2023.”
"We're moving forward steadily with our ambition to provide improved, multiplexed TCR-Ts to a wide patient base," said Gavin MacBeath, Ph.D., Chief Executive Officer. "The IND endorsement of TSC-203-A0201, the fourth TCR-T cell product licensed for our T-Plex solid tumor program, emphasizes the power of our discovery platform and the prospective life-saving capability of cell therapy in treating various solid tumors. PRAME, owing to its high prevalence in solid tumors and promising results in recent clinical trials, is particularly significant. Concurrently, we are expanding our efforts to increase the quantity of therapeutic TCRs fit for clinical use and expect additional IND submissions for our solid tumor program.”
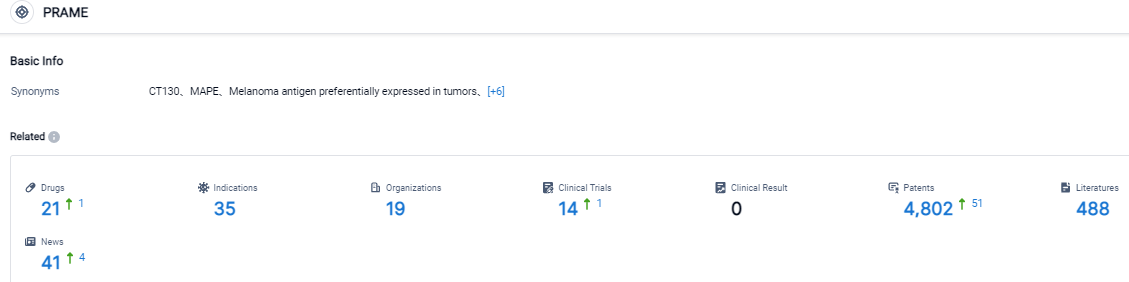
Click on the image below for direct access to the latest R&D progress on PRAME target drugs, indications, research institutions, clinical trials, and more. As of August 31, 2023, there are 21 investigational drugs for the PRAME target, including 35 applicable indications, 19 R&D institutions involved, with related clinical trials reaching 14, and as many as 4802 patents. TScan is a biopharmaceutical firm in the clinical stage, working on the evolution of T cell receptor engineered T cell treatments for cancer patients. The prime TCR-T therapy contenders of the firm, TSC-100 and TSC-101, are under progress for eradicating residual disease and stopping relapse post allogeneic hematopoietic cell transplantation in patients suffering from hematologic malignancies. Moreover, the firm is also advancing multiplexed TCR-T therapy contenders for treating numerous solid tumors. The company has designed and is consistently building its ImmunoBank, which is a repository for therapeutic TCRs recognizing varied targets and linked with multiple HLA types. The goal is to provide personalized multiplexed TCR-T therapies for patients suffering from assorted solid tumors.