AbbVie's JAK inhibitor, Upadacitinib Extended-Release Tablet, has been approved in China for two new indications, the treatment of nr-axSpA and AS
Recently, AbbVie announced that the National Medical Products Administration (NMPA) of China has approved two new indications for its JAK inhibitor, upadacitinib extended-release tablets. They are for adult patients with active radiographic axial spondyloarthritis (nr-axSpA) with poor response to Non-Steroidal Anti-Inflammatory Drugs (NSAID) and objective signs of inflammation, and for adult patients with active ankylosing spondylitis (AS) with poor response or intolerance to one or more TNF inhibitors. Upadacitinib extended-release tablets have become the first oral targeted drug in China to cover the entire course of axial spondyloarthritis (axSpA).
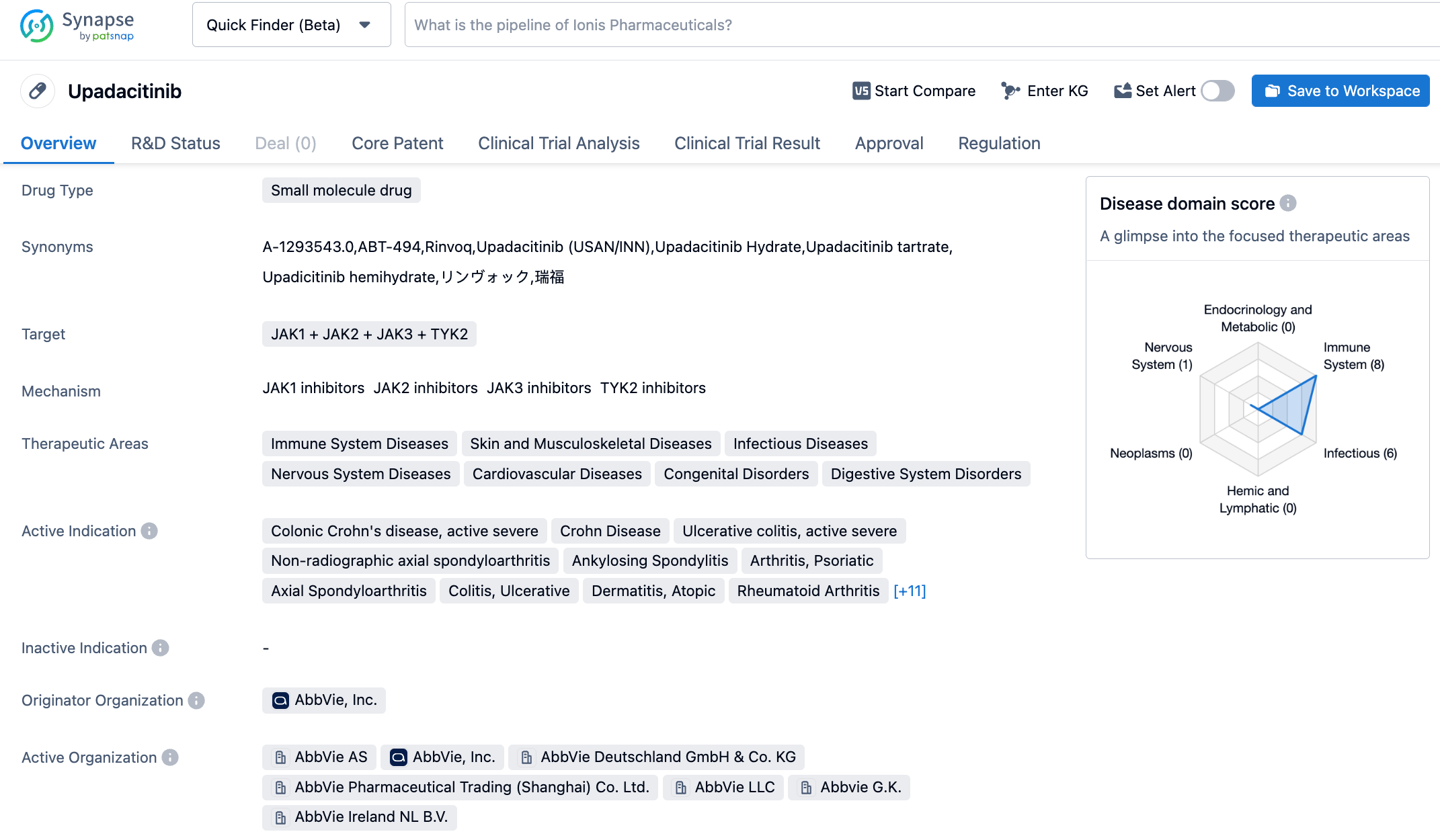
Upadacitinib is a once daily, selective, and reversible JAK1 inhibitor, which has significantly more inhibitory potency towards JAK1 than JAK2, JAK3, and TYK2. The drug was first approved in the United States in August 2019, for the treatment of rheumatoid arthritis and in China in February 2022, for adult and adolescent patients aged 12 and above with moderate to severe atopic dermatitis who are candidates for systemic therapy. The JAK family is a group of non-receptor tyrosine kinases, including four subtypes: JAK1, JAK2, JAK3, and TYK2, which play an important role in the signal cascade of various type I and II cytokine receptors. The JAK-mediated signaling pathway is associated with cell proliferation, differentiation, apoptosis, and inflammation. JAK1 plays an important role in the pathophysiological process of immune-mediated diseases, which makes JAK1 inhibitors have potential to treat a variety of diseases. To date, upadacitinib has seven approved indications and is currently being studied in several immune-mediated diseases.
The NMPA's approval of upadacitinib extended-release tablets for the treatment of nr-axSpA is based on data from the phase 3 SELECT-AXIS 2 clinical trial. The study evaluated the efficacy, safety, and tolerability of the product in adult patients with active nr-axSpA. The results showed that compared with placebo, nearly half of the patients receiving 15mg upadacitinib extended-release tablets achieved an ASAS40 response (primary endpoint) by week 14 (44.9% versus 22.3%). In those receiving upadacitinib extended-release tablets, an ASAS40 response was observed as early as two weeks into treatment.
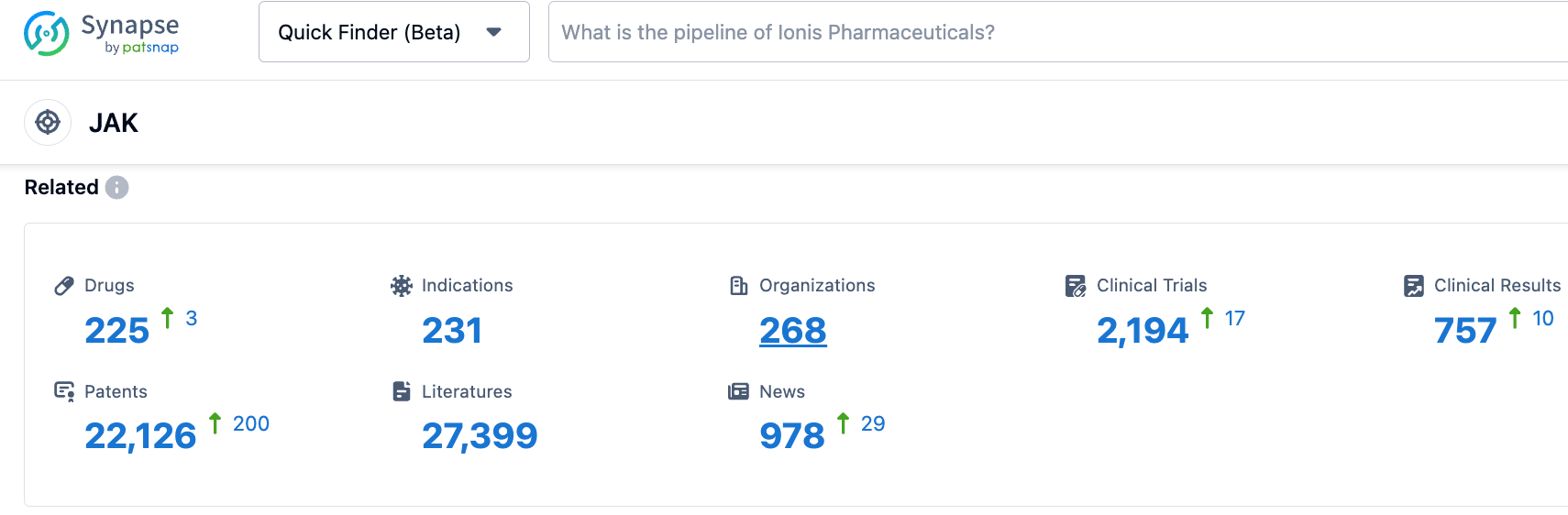
According to the Synapse database, as of October 28, 2023, there are 224 JAK-targeting drugs in development, covering 229 indications, from 268 institutions, involving 2191 related clinical trials, and as many as 22031 patents. The market for JAK inhibitors reached nearly $10 billion in 2022. Due to its first-mover advantage, the first-generation pan-JAK inhibitors still occupy the dominant market position, while the second-generation JAK inhibitors led by AbbVie, such as upadacitinib, are gradually eroding the market share of the first-generation JAK inhibitors. The global sales of upadacitinib reached $2.522 billion in 2022, which was very impressive. According to AbbVie's forecast, the sales of upadacitinib will exceed $7.5 billion by 2025.