ExploringEbastine's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Ebastine's R&D Progress
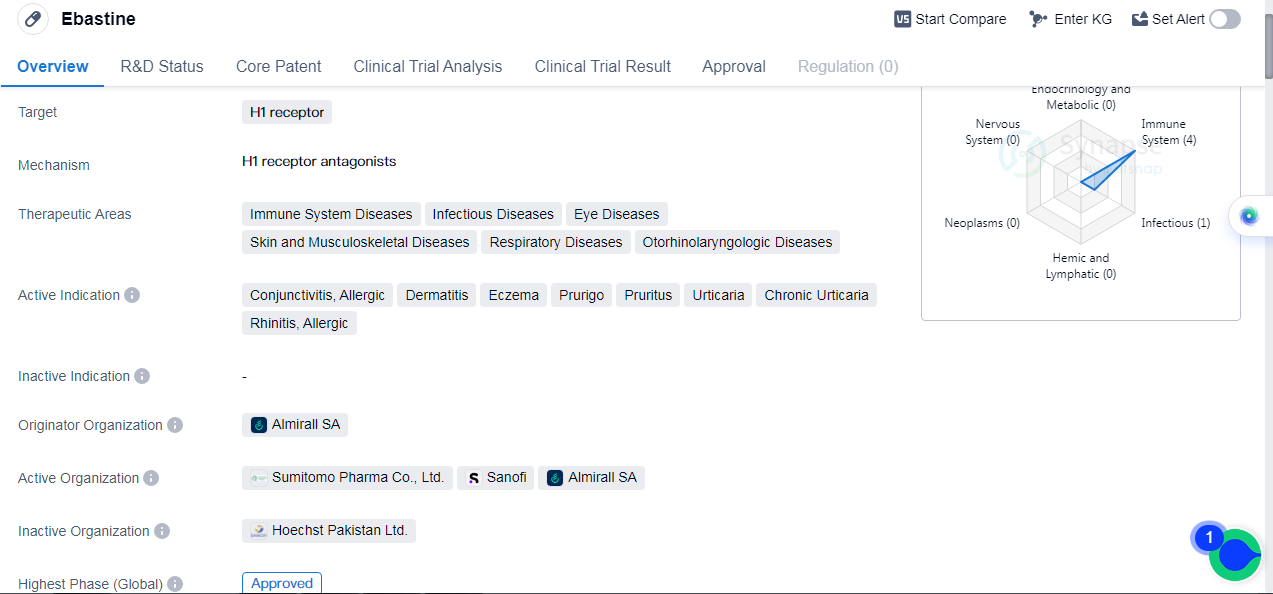
Ebastine is a small molecule drug that targets the H1 receptor. It has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, ocular diseases, skin and musculoskeletal diseases, respiratory diseases, and otorhinolaryngologic diseases. The drug is indicated for the treatment of conjunctivitis, allergic dermatitis, eczema, prurigo, pruritus, urticaria, chronic urticaria, and allergic rhinitis.
Ebastine was first approved in Spain in January 1990 by Almirall SA, the originator organization. It has since received approval in other countries globally, making it available for patients worldwide. In China, it has also obtained approval, indicating its recognition and acceptance in the Chinese market.
As a small molecule drug, Ebastine is designed to interact with the H1 receptor, which is involved in allergic responses. By targeting this receptor, the drug aims to alleviate symptoms associated with various allergic conditions, such as itching, inflammation, and redness.
The approval of Ebastine in multiple therapeutic areas highlights its versatility and potential to address a wide range of medical conditions. Immune system diseases, infectious diseases, ocular diseases, skin and musculoskeletal diseases, respiratory diseases, and otorhinolaryngologic diseases can all benefit from the therapeutic effects of Ebastine.
Conjunctivitis, allergic dermatitis, eczema, prurigo, pruritus, urticaria, chronic urticaria, and allergic rhinitis are among the active indications for Ebastine. These conditions are characterized by allergic reactions and inflammation, which can cause discomfort and impair the quality of life for affected individuals. Ebastine offers a treatment option to alleviate these symptoms and improve patient well-being.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ebastine: H1 Receptor Antagonists
H1 receptor antagonists, also known as H1 blockers or antihistamines, are a class of drugs that block the action of histamine at the H1 receptors. Histamine is a chemical released by the body during an allergic reaction, causing symptoms like itching, sneezing, runny nose, and watery eyes. H1 receptor antagonists work by binding to the H1 receptors on cells and preventing histamine from attaching to these receptors. This blocks the histamine-mediated allergic response and helps alleviate symptoms associated with allergies. H1 receptor antagonists are commonly used to treat allergic conditions such as hay fever, allergic rhinitis, hives, and allergic conjunctivitis. They can also be used to manage symptoms of non-allergic conditions like motion sickness and insomnia.
Drug Target R&D Trends for Ebastine
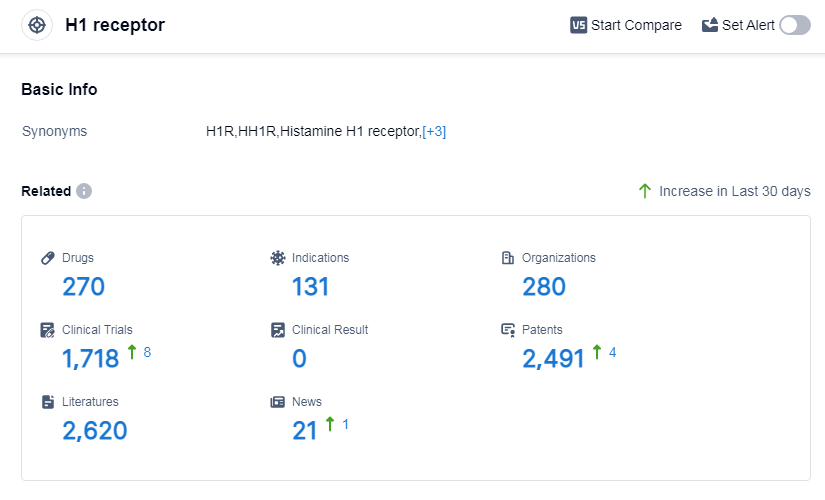
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 270 H1 receptor drugs worldwide, from 280 organizations, covering 131 indications, and conducting 1718 clinical trials.
The analysis of the target H1 receptor reveals a competitive landscape with multiple companies actively involved in research and development. GSK Plc, Sanofi, and Bayer AG are the companies growing fastest under this target, with GSK Plc having the highest stage of development. The most common indications for drugs under the target H1 receptor include rhinitis, allergic, common cold, urticaria, pruritus, and hypersensitivity. Small molecule drugs are progressing most rapidly, indicating intense competition in this area. China is leading in terms of development, followed by the United States and Japan. The future development of the target H1 receptor is expected to continue with a focus on small molecule drugs and expanding indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Ebastine is a well-established small molecule drug that has been approved for use in various countries globally, including China. Its approval in multiple therapeutic areas and active indications demonstrates its potential to address a wide range of allergic conditions. As an expert in the pharmaceutical industry, it is important to consider the potential market opportunities and patient benefits that Ebastine can offer in the field of biomedicine.