AltruBio declares FDA approval on IND submission for ALTB-268, an Immune Checkpoint Booster, triggering a Phase 2 Clinical Study for dealing with Ulcerative Colitis
AltruBio Inc., a biotechnology organization focused on the creation of innovative treatments for immune-related diseases with considerable healthcare necessities, unveiled that its IND request has been accepted by the U.S. FDA. This permission allows the company to begin Phase 2 clinical trials for its under-the-skin applied immune checkpoint booster, ALTB-268, targeting ulcerative colitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We are excited to progress our subcutaneous ICE, ALTB-268, into Phase 2 trials. This marks a substantial step in advancing our innovative PSGL-1 agonist antibodies in the clinical stage, bringing them one stage closer to needy patients," stated Judy Chou, AltruBio's President and CEO.
Chou further elaborated, "AltruBio has made impressive leaps over the past year in achieving our clinical objectives, such as concluding our Phase 1 trial successfully and releasing favorable top-line data for ALTB-268. We hold tremendous faith in our groundbreaking strategy that naturally reinstates immune stability and treats immune disorders at their root. We anticipate starting research on patients with ulcerative colitis by the end of 2023."
The Phase 2 experiments include an open-label preliminary biomarker clinical trial of ALTB-268 in patients with biologics-resistant ulcerative colitis and a randomized global multi-arm research investigating two active dosages of ALTB-268 alongside placebo. The preliminary biomarker study is projected to kick-off by the end of 2023, and enrollment for the randomized trial is expected to begin in 2024.
With its action like an immune checkpoint enhancer, ALTB-268 is a tetravalent PSGL-1 agonist antibody. It selectively downregulates chronically activated T-cells by blocking the T-cell effector function and thus fosters T-cell exhaustion and apoptosis. ALTB-268 aids in rebalancing the immune system without systemic suppression and treats immune diseases at their root.
ALTB-268 displayed enhanced effectiveness in preclinical studies and is designed for subcutaneous administration, making it patient-friendly. Owing to the relevancy of T-cell modulation in numerous immunological diseases, ALTB- 268 provides a broad potential for expansion in various indications, making it a promising "pipeline-in-a-product."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
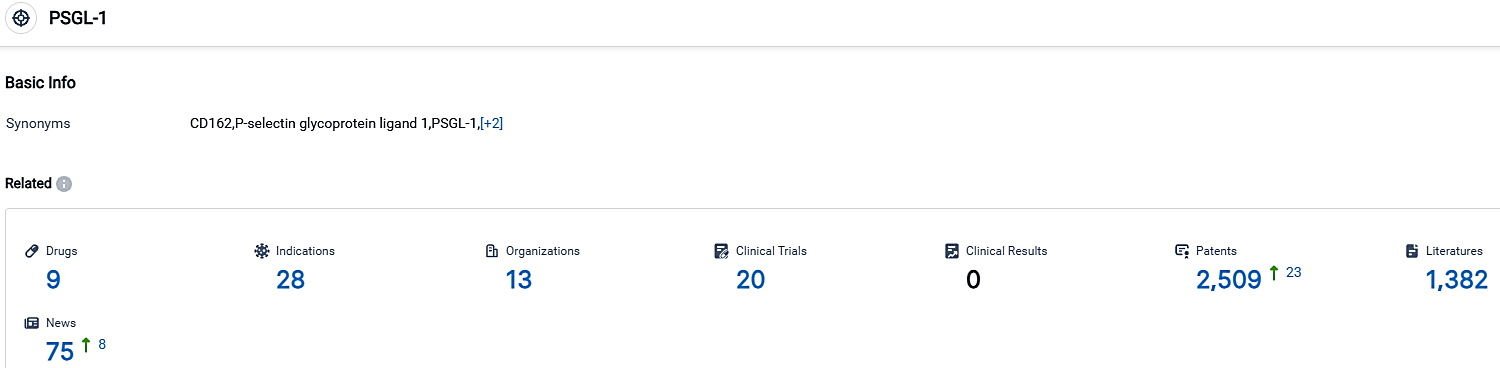
According to the data provided by the Synapse Database, As of October 3, 2023, there are 9 investigational drugs for the PSGL-1 target, including 28 indications,13 R&D institutions involved, with related clinical trials reaching 20,and as many as 2509 patents.
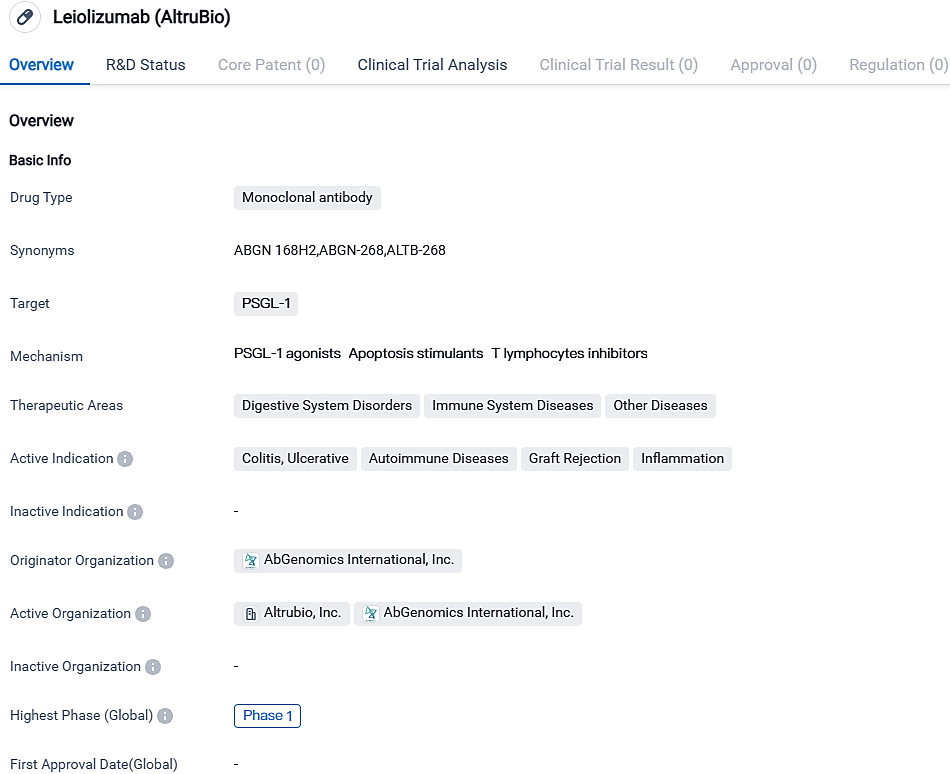
ALTB-268, the monoclonal antibody zooming in on PSGL-1, carries potential treatment implications for conditions like colitis, ulcers, autoimmune disorders, graft rejection, and inflammation. At present, it's undergoing Phase 2 development, necessitating more investigation and therapeutic studies to ascertain its safety and therapeutic effectiveness for these ailments.