AC Immune Set to Reacquire Worldwide Ownership of Crenezumab and Semorinemab
AC Immune SA, a biopharmaceutical firm at the forefront of developing targeted therapies for neurological disorders, has disclosed that following the conclusion of their partnership with Genentech—a Roche Group affiliate—and Roche itself, the enterprise will reclaim complete international ownership of two significant therapeutic antibodies: the anti-amyloid beta monoclonal antibody known as crenezumab and the anti-Tau monoclonal antibody referred to as semorinemab. These particular antibodies have undergone rigorous testing in several clinical trials concerning the potential treatment of Alzheimer's disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
AC Immune is set to reacquire the rights to pre-existing GMP-grade drug products destined for clinical trials, together with the relevant information compiled through previous agreements. AC Immune is on track to methodically inspect and consider the data sets at hand, which include conclusive outcomes from the open-label extension phase of the Lauriet study. This review will take place once AC Immune wholly obtains the data and prior to making any strategic decisions regarding the further progression of these products or exploring new avenues.
Dr. Andrea Pfeifer, the Chief Executive Officer of AC Immune SA, remarked: "Our candidates currently under development are part of active Phase 2 clinical studies that could contribute to their registration. This includes the newly inaugurated prevention trial for individuals who have yet to display symptoms of Alzheimer's disease. Our innovative strategy, leveraging the patient's immune system to delay and hopefully prevent the arrival of neurodegenerative illnesses, stands to reshape our approach to treating these afflictions."
"With our regained complete control over crenezumab, semorinemab, and the associated intellectual property, we are opening doors to potential strategic pathways and combination treatments, encouraging new avenues for progress. Our belief is steadfast in the enhanced capabilities of these programs, armed with full rights and insights gained, through the integration of AC Immune’s pioneering technologies. Our community can expect to see emerging data later this year that will unveil the expansive capabilities of these monoclonal antibody assets."
Crenezumab works as a humanized monoclonal antibody, a novel therapeutic candidate aimed at lessening the advancement of Alzheimer’s Disease (AD) by targeting neurotoxic beta-amyloid oligomers. This antibody was crafted by AC Immune to specifically engage with various aberrant structures of Abeta. Additionally, its antibody framework has been engineered to reduce brain inflammation, which could lower the occurrence of adverse side effects such as ARIA.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
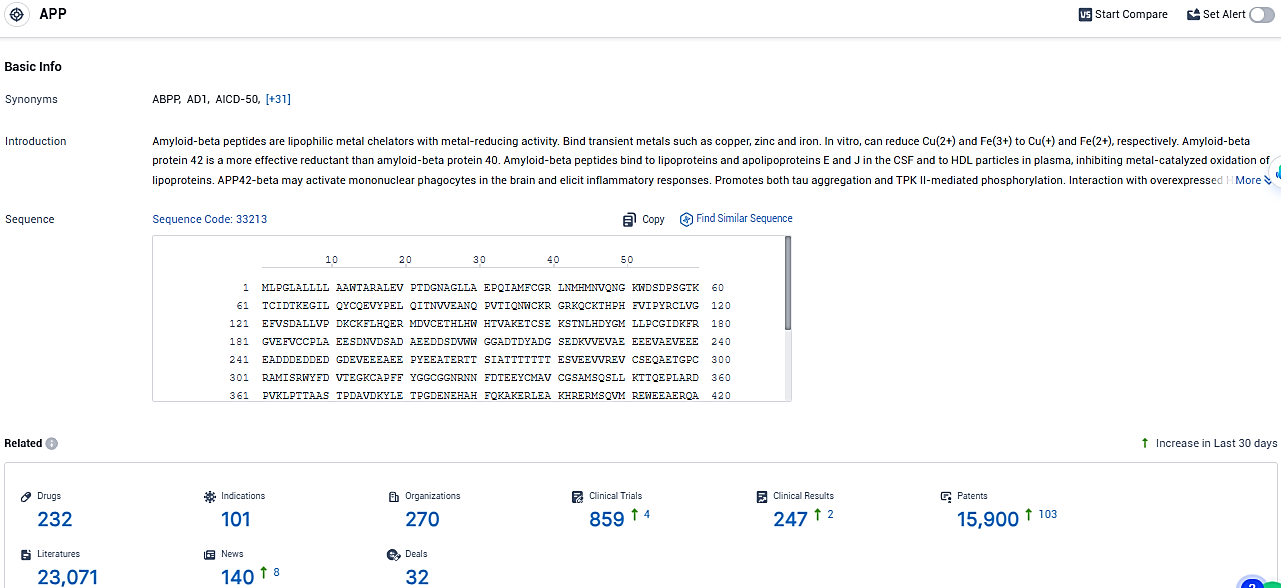
According to the data provided by the Synapse Database, As of January 26, 2024, there are 232 investigational drugs for the APP target, including 101 indications, 270 R&D institutions involved, with related clinical trials reaching 859, and as many as 15900 patents.
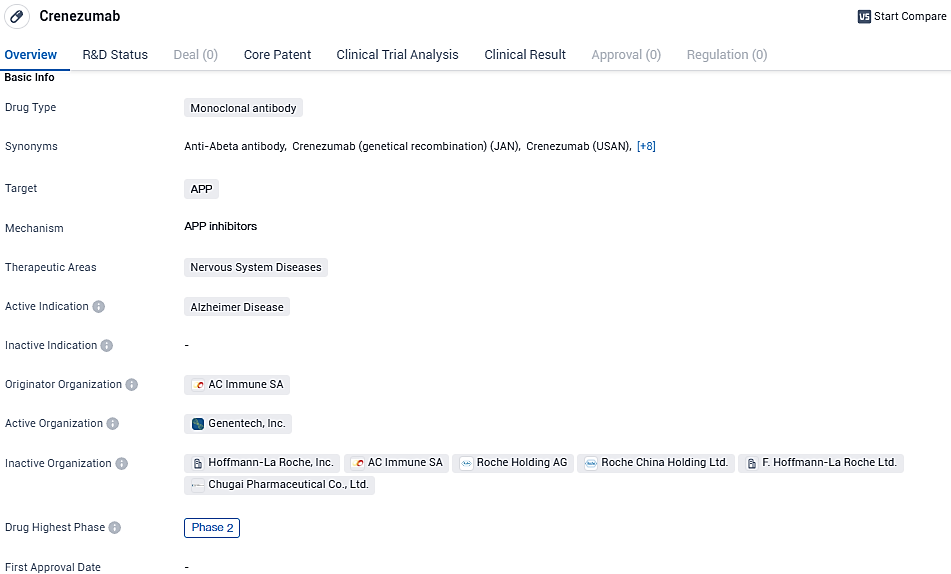
Crenezumab is a monoclonal antibody drug being developed by AC Immune SA to target APP in the treatment of Alzheimer's disease. It is currently in Phase 2 of clinical development globally and falls under the therapeutic area of nervous system diseases. While it shows promise in its potential to address the unmet medical need in Alzheimer's disease, its development in China has been suspended.