Ionis Reports Promising Preliminary Results in Phase 3 of OASIS-HAE Trial on Donidalorsen for Genetic Angioedema
Ionis Pharmaceuticals, Inc. has reported favorable preliminary findings from its Phase 3 OASIS-HAE trial assessing the efficacy of donidalorsen for individuals suffering from hereditary angioedema. The study achieved its main goal, which was to demonstrate a decrease in the frequency of angioedema episodes among participants who received subcutaneous injections of donidalorsen, administered either monthly or bimonthly, in contrast to those given a placebo.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Moreover, clinical evaluations revealed that donidalorsen significantly met both secondary objectives in the every four week (Q4W) cohort and crucial secondary objectives in the every eight week (Q8W) cohort. The safety and tolerability of donidalorsen were found to be commendable during the research, with no instances of severe adverse events occurring among individuals receiving the treatment.
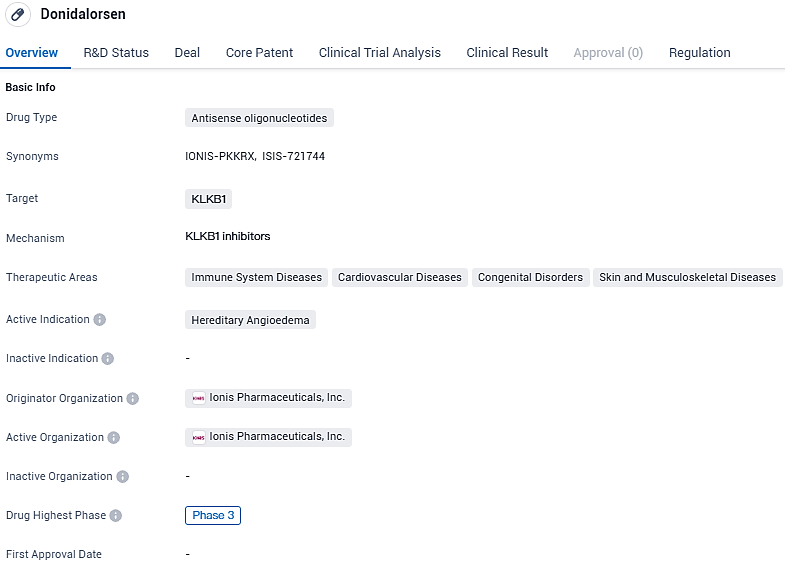
Hereditary angioedema (HAE) is a genetically inherited condition that is both rare and potentially fatal, characterized by spontaneous and recurring episodes of pronounced swelling affecting the skin, gastrointestinal system, upper respiratory tract, as well as the face and throat areas. Donidalorsen, currently undergoing investigation, is an RNA-targeted prophylactic therapeutic agent devised with the capacity to accurately inhibit the production of prekallikrein. This disruption halts the process that leads to HAE flare-ups.
Reflecting on this evidence, Ionis is progressing towards the submission of a New Drug Application to the U.S. Food and Drug Administration. Otsuka, holding the exclusive commercialization rights for donidalorsen in Europe, is likewise moving forward with plans to file a Marketing Authorization Application with the European Medicines Agency. The drug has been designated as an Orphan Drug in the U.S., while the designation process is in progress within the EU.
Kenneth Newman, M.D., Ionis's Senior Vice President and Head of Clinical Development, expressed enthusiasm about the encouraging primary findings from the Phase 3 OASIS-HAE study involving donidalorsen.
Dr. Newman elaborated, "Given these findings, combined with the sustained efficacy and positive safety profile seen in the current Phase 2 open-label extension study, we envision donidalorsen as a potentially valuable new therapy for individuals with HAE. These patients often suffer from acute, distressing, and intense breakthrough bouts even when on existing prophylactic measures. We extend our heartfelt appreciation to the participants, their support networks, the medical professionals, and research teams engaged in the OASIS-HAE study."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
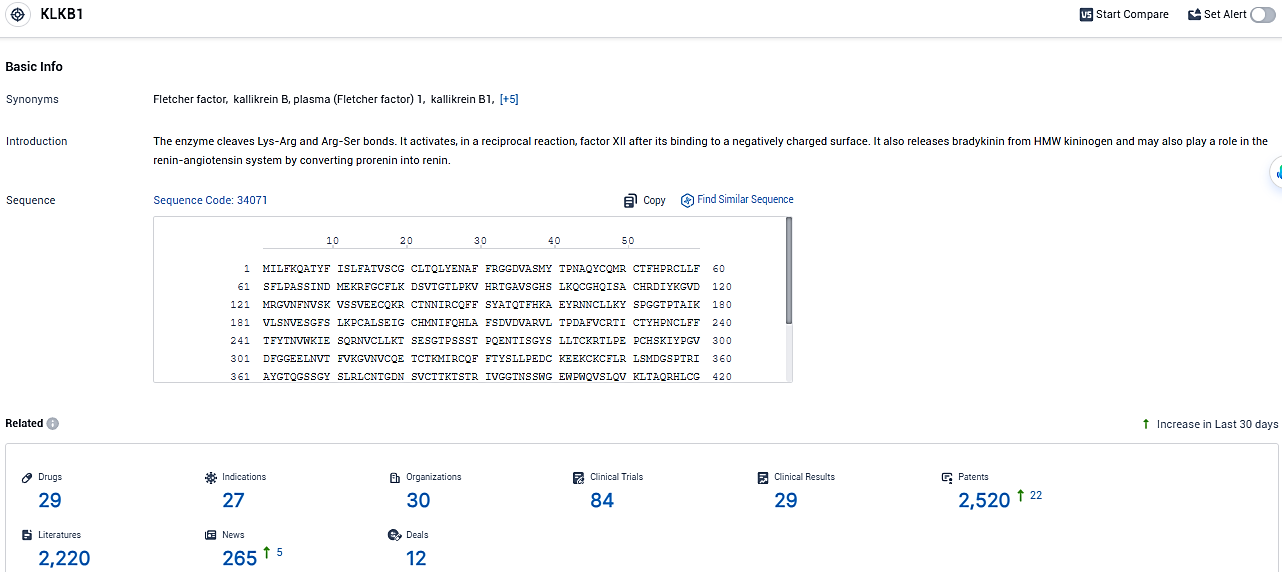
According to the data provided by the Synapse Database, As of January 26, 2024, there are 29 investigational drugs for the KLKB1 target, including 27 indications, 30 R&D institutions involved, with related clinical trials reaching 84, and as many as 2520 patents.
Donidalorsen targets the KLKB1 protein and aims to treat hereditary angioedema, a rare genetic disorder. The drug is currently in Phase 3 of clinical development and has been granted orphan drug status.