EMA Accepts Submission for Linvoseltamab as Therapy for Recurrent/Resistant Multiple Myeloma
Regeneron Pharmaceuticals, Inc. has disclosed that the European Medicines Agency is currently examining their submitted Marketing Authorization Application, which pertains to the therapeutic agent linvoseltamab. This treatment is specifically designed for use in adult individuals who are battling relapsed or resistant multiple myeloma, and have experienced further disease progression following a minimum of three previous treatment attempts.
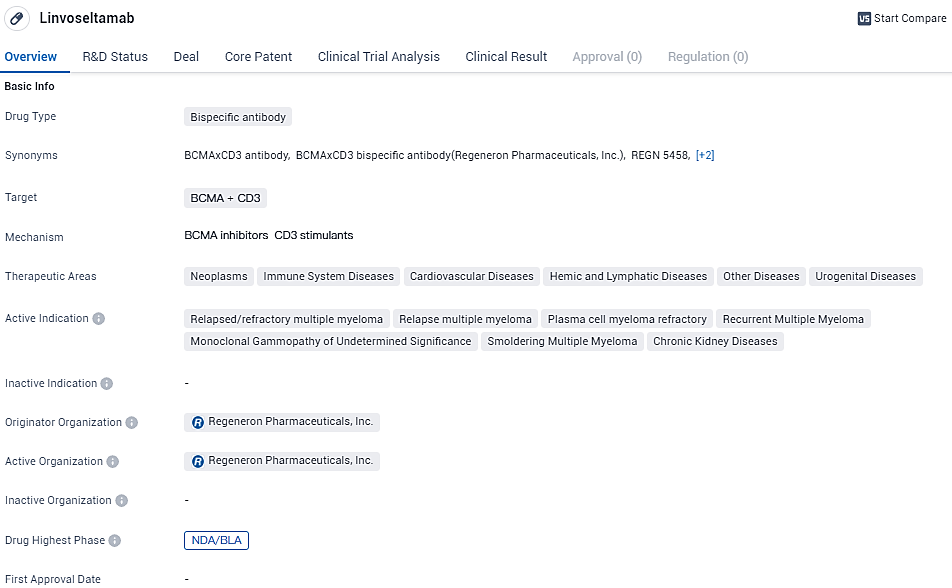
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Linvoseltamab is an investigational bispecific antibody designed to bridge B-cell maturation antigen
Linvoseltamab is a novel bispecific antibody under research that has been engineered to connect the B-cell maturation antigen (BCMA) found on multiple myeloma cells to T cells containing the CD3 receptor. This connection is meant to prompt the activation of T cells and the subsequent destruction of the cancerous cells.
Data underpinning the MAA involve results from a preliminary Phase 1/2 key study focused on assessing linvoseltamab for patients with relapsed or refractory multiple myeloma (R/R MM). These findings were disclosed in the final month of 2023. Concurrently, a Biologics License Application for linvoseltamab was lodged with the FDA that same December.
As a major hematological malignancy, multiple myeloma accounts for over 176,000 fresh diagnoses worldwide each year. The disease primarily consists of abnormal plasma cells multiplying and outcompeting normal blood cells in the bone marrow, while also spreading to and compromising other body tissues, risking severe organ damage. Despite medical progress, MM remains an incurable illness. Though existing therapies can decelerate its advancement, the majority of patients will nonetheless see their illness progress and will be in need of further treatment options.
Within the clinical studies for linvoseltamab, an ongoing Phase 3 confirmatory trial is actively recruiting participants.
Further research efforts are either organized or in the process, aiming to explore treatment in initial therapy sessions and different stages of the disease. This includes a Phase 1/2 study for first-line therapy, a Phase 2 study for patients with high-risk smoldering multiple myeloma, and another Phase 2 study focusing on monoclonal gammopathy of undetermined significance. Moreover, there are plans for a Phase 1 study that will test linvoseltamab alongside a CD38xCD28 costimulatory bispecific antibody in multiple myeloma patients.
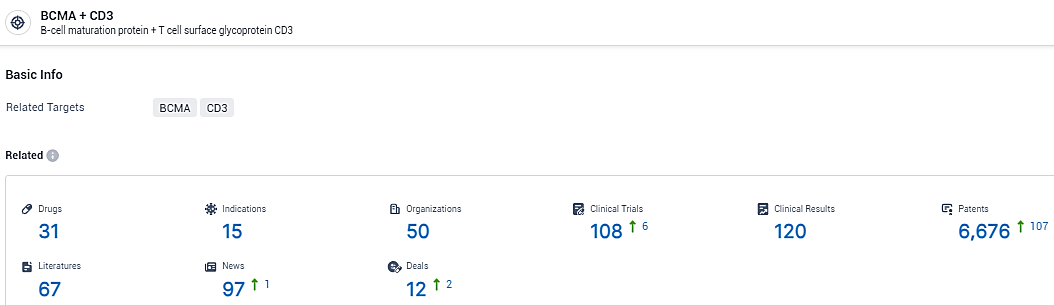
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 8, 2024, there are 31 investigational drugs for the BCMA and CD3 target, including 15 indications, 50 R&D institutions involved, with related clinical trials reaching 108, and as many as 6676 patents.
Linvoseltamab is a bispecific antibody drug targeting BCMA and CD3, primarily indicated for relapsed/refractory multiple myeloma. Its originator organization is Regeneron Pharmaceuticals, Inc., and it is currently in the NDA/BLA phase. The drug's potential therapeutic areas extend beyond multiple myeloma, but further research is needed to explore its efficacy and safety in these areas. Monitoring its progress and regulatory approvals will be crucial for assessing its impact on the pharmaceutical industry and patients.