4DMT's Study Shows Promising Outcomes for Eye Injection 4D-150 in Treating Wet Age-Related Macular Degeneration
A prominent biotechnology firm in the clinical phase, 4D Molecular Therapeutics, which specializes in developing genetic medical treatments aimed at effectively treating common diseases with significant market size, has shared encouraging early results from the ongoing Phase 2 PRISM study. This trial is investigating the effects of the intravitreal injection of 4D-150 in individuals diagnosed with the advanced stages of wet age-related macular degeneration, particularly those experiencing intense disease progression and requiring frequent treatment.
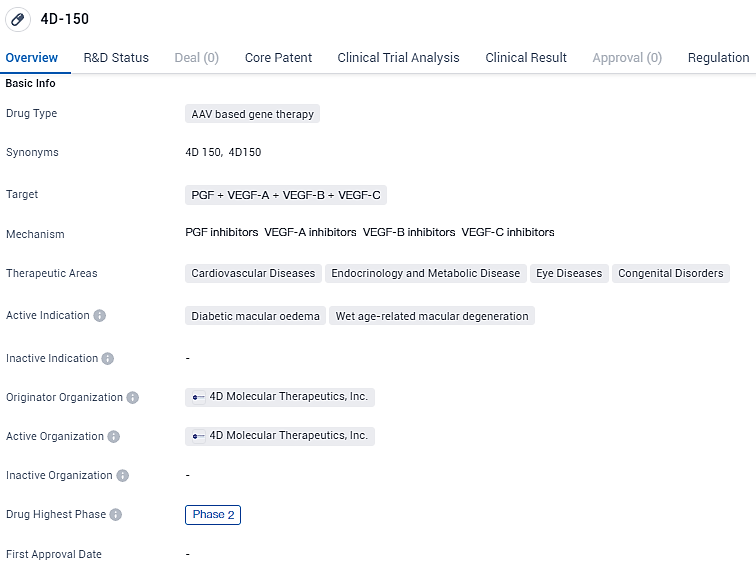
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
At the recent gathering for the Angiogenesis, Exudation, and Degeneration 2024 Conference, Arshad M. Khanani, M.D., M.A., FASRS, showcased pivotal findings at the 24-week juncture from the Phase 2 Dose Expansion group within the PRISM clinical study.
David Kirn, M.D., co-creator and CEO of 4DMT, expressed excitement over the preliminary positive data, suggesting the promising capabilities of 4D-150 as an effective, user-friendly, long-lasting, and revolutionary intravitreal solution for those suffering from wet age-related macular degeneration (AMD), aiming to maintain visual function over an extended period.
"One of the exciting aspects we are observing is the potential of 4D-150 to revolutionize the current approach to treating these patients, and these findings further emphasize the strength and contribution of our proprietary intravitreal R100 vector developed by 4DMT. I extend my gratitude to all the patients and investigators involved in the PRISM study who have contributed to reaching this significant achievement," Kirn further stated.
Dr. Robert Kim, the Chief Medical Officer, remarked on the encouraging Phase 2 interim outcomes, emphasizing that they reveal a unique treatment profile for wet AMD, particularly for patient groups that have been historically underserved in previous research efforts.
Dr. Kim also mentioned, "Moving forward, we are eager to engage with regulatory bodies to finalize a Phase 3 trial strategy accelerated by the FDA’s RMAT and the EMA’s PRIME designations. Our goal is to expedite the progress of 4D-150 and offer a potent new therapeutic alternative for the millions affected by ocular diseases associated with VEGF, which often lead to vision loss."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 8, 2024, there are 1 investigational drugs for the PGF and VEGF-A and VEGF-B and VEGF-C target, including 2 indications, 1 R&D institutions involved, with related clinical trials reaching 2, and as many as 134 patents.
4D-150 targets multiple growth factors and is being investigated for its potential in treating various diseases, including diabetic macular oedema and wet age-related macular degeneration. Currently in Phase 2, the drug has received regulatory designations such as RMAT and PRIME, which aim to facilitate its development.