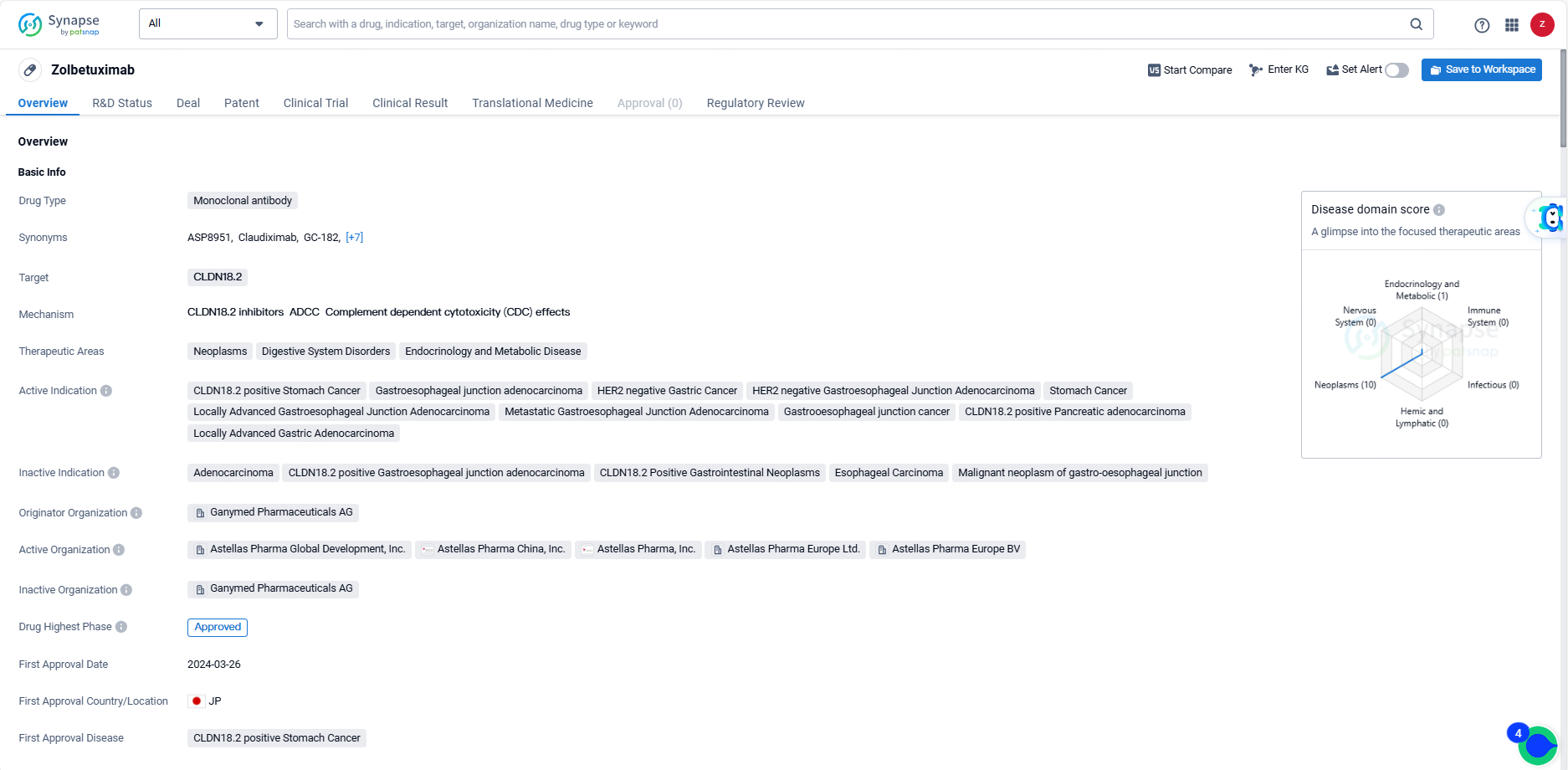
FDA Acknowledges Astellas' Resubmitted Zolbetuximab Application, Sets New Target Date
Astellas Pharma Inc. revealed that the U.S. Food and Drug Administration has accepted the company's resubmission of the Biologics License Application for zolbetuximab. Zolbetuximab is a pioneering investigational monoclonal antibody targeting claudin (CLDN) 18.2, intended for the initial treatment of adults with CLDN18.2-positive, locally advanced unresectable or metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric or gastroesophageal junction adenocarcinoma.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Should zolbetuximab receive approval, it would become the inaugural CLDN18.2-targeted therapy available for this patient demographic in the U.S. Under the Prescription Drug User Fee Act, the FDA has established a new expected action date of November 9, 2024.
In the United States, projections for 2024 indicate that 26,890 individuals will receive a gastric cancer diagnosis, with 10,880 fatalities expected. Early-stage gastric cancer symptoms often resemble common gastrointestinal issues, leading to diagnoses frequently occurring at advanced or metastatic stages—when the cancer has extended beyond its original site to additional tissues or organs. The relative five-year survival rate for those with metastatic gastric cancer stands at 7%.
"Astellas is devoted to delivering innovative targeted therapies for challenging cancer types. Patients with advanced gastric or GEJ cancer frequently encounter significant unmet needs. The FDA’s recognition of the zolbetuximab BLA resubmission is a crucial step towards providing this vital treatment option to eligible patients in the U.S. battling this lethal disease," stated Moitreyee Chatterjee-Kishore, Ph.D., M.B.A., Senior Vice President and Head of Immuno-Oncology Development at Astellas.
The zolbetuximab BLA was resubmitted on May 9, 2024, after the FDA issued a complete response letter on January 4, 2024, highlighting third-party manufacturing deficiencies found during the pre-license facility inspection. The FDA did not raise any questions regarding the clinical data’s efficacy or safety and did not request additional clinical studies to support the BLA’s approval.
The zolbetuximab BLA is founded on data from the Phase 3 SPOTLIGHT and GLOW clinical trials. The SPOTLIGHT trial assessed zolbetuximab plus mFOLFOX6 versus placebo plus mFOLFOX6. The GLOW trial investigated zolbetuximab plus CAPOX (a chemotherapy regimen comprising capecitabine and oxaliplatin) against placebo plus CAPOX.
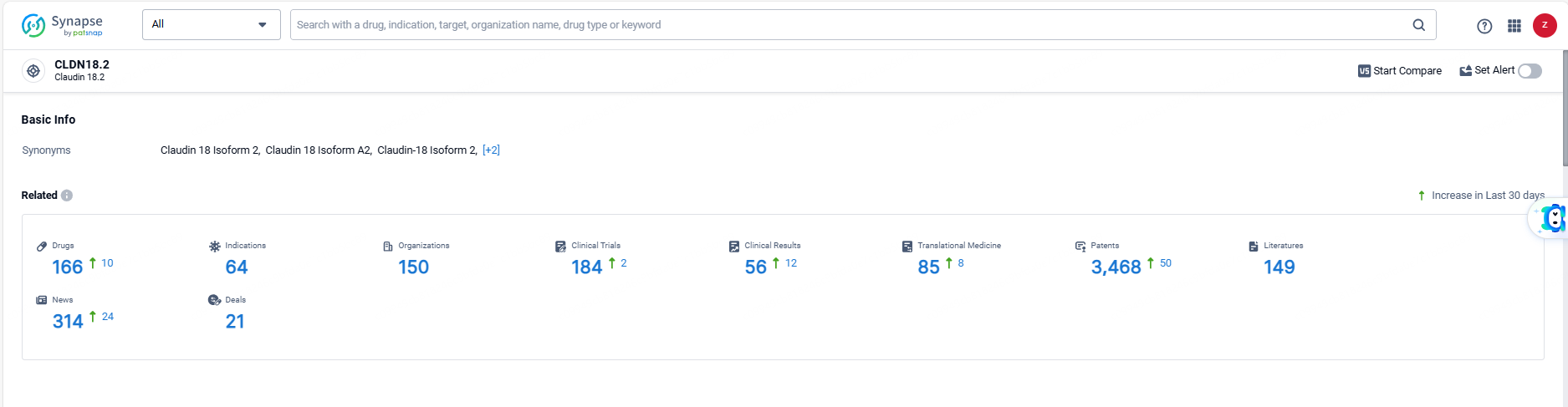
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 7, 2024, there are 166 investigational drugs for the CLDN18.2 target, including 64 indications, 150 R&D institutions involved, with related clinical trials reaching 184, and as many as 3468 patents.
Zolbetuximab's approval and regulatory status reflect its potential as a valuable addition to the treatment options available for patients with the specified types of cancer. Its development and approval represent a significant advancement in the field of biomedicine and the pharmaceutical industry's ongoing efforts to address complex and challenging diseases.