AcuraStem confirms licensing contract with Takeda to progress PIKFYVE treatment methods
AcuraStem, a biotech firm with a patient-oriented approach revolutionizing the development of therapies for neurodegenerative disorders, proclaimed it has signed a licensing deal with Takeda. The deal involves the progression and marketing of AcuraStem's PIKFYVE-targeted treatments, including the groundbreaking antisense oligonucleotide, AS-202, designed specifically for Amyotrophic Lateral Sclerosis (ALS) management.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
PIKFYVE has been identified as a new therapeutic objective for ALS and may also be important for the treatment of other TDP-43 proteinopathies such as Frontotemporal Dementia. AcuraStem’s therapeutic methodology for PIKFYVE is aimed at battling neurodegeneration by eliminating toxic protein clusters and safeguarding healthy neuronal processes.
This unique therapeutic approach was originally unearthed in Justin Ichida's, PhD, laboratory, AcuraStem's co-founder, by using disease models derived from patients, and AcuraStem holds the sole license from the USC Stevens Center for Innovation. The mechanism was further advanced on AcuraStem's iNeuroRx® disease modelling platform.
Researchers at AcuraStem detailed that ASO-mediated suppression of PIKFYVE has the potential to invigorate motor function, minimize neurodegeneration, and escalate survival in multiple in vivo models of both ALS and FTD.
As per the agreement, Takeda will obtain an exclusive global license for AcuraStem’s PIKFYVE project. AcuraStem is set to receive a sum of approximately $580 million in upfront and milestone payments if all upcoming clinical, regulatory, and commercial objectives are met throughout the agreement's term. Additionally, they will receive tiered royalties on potential net sales resulting from any commercial products issued from this license.
AcuraStem will carry out specific activities to assist in the progression of AS-202 IND enabling studies and characterizing probable backup ASOs. Takeda will handle remaining development activities which include clinical development, regulatory affairs, and worldwide commercialization.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
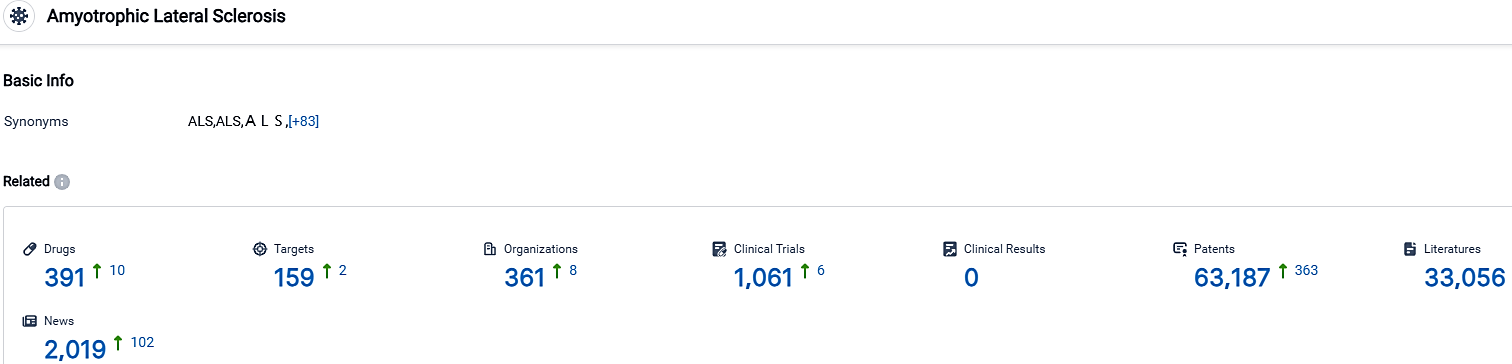
According to the data provided by the Synapse Database, As of October 1, 2023, there are 391 investigational drugs for the Amyotrophic Lateral Sclerosis, including 159 targets,361 R&D institutions involved, with related clinical trials reaching 1061,and as many as 63187 patents.
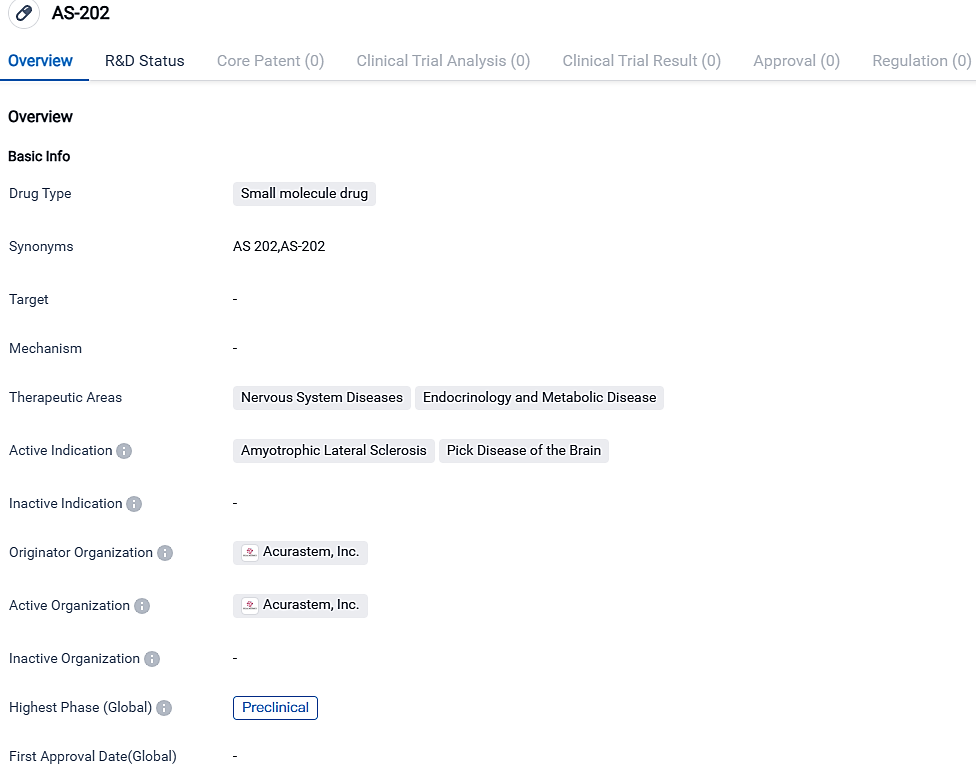
Acurastem, Inc. is in the process of developing a small molecule medicine known as AS-202, targeted at treating Amyotrophic Lateral Sclerosis and Pick Disease of the Brain. At present, the drug is in the preclinical stage with laboratory evaluations underway to determine its safety and efficacy. The active progression of AS-202 underscores the continued commitment in the realm of pharmaceuticals to discover therapeutic prospects for neurodegenerative disorders.