RAS Inhibitors: A New Weapon Targeting a Deadly Factor in Cancer
RAS is a small G protein composed of 188 amino acids, primarily distributed on the inner membrane of the cytoplasm in cells, its activity undergoes dynamic changes. When RAS is bound to GDP, it is in an inactive state, however, when RAS binds with GTP, it is in an activated state, thereby promoting the activation of a variety of downstream signaling pathways, such as MAPK, PI3K, RAF, etc. A variety of RAS mutations can cause it to be constantly in an active state, such overactivation promotes the development of cancer, thus RAS inhibitors can be regarded as a sharp sword to inhibit tumors.
RAS protein has three subtypes, KRAS, HRAS, NRAS, among which the frequency of KRAS mutations accounts for 85% of the total number of RAS mutations, NRAS accounts for 11%, and HRAS only 4%. The most common mutation in KRAS is the G12 mutation, including G12D, G12C, G12V. Research shows that these mutations can cause RAS to be constantly bound to GTP, and remain in an active state. Various data websites all show that RAS mutations can promote the development of many types of tumors. Although the carcinogenic mechanism of KRAS is relatively clear, KRAS was deemed "undruggable" because it has no obvious binding sites in its structure, and research on inhibitors that directly target KRAS did not make any breakthroughs for a long time.
Ras Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 25 Sep 2023, there are a total of 279 Ras drugs worldwide, from 217 organizations, covering 71 indications, and conducting 250 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.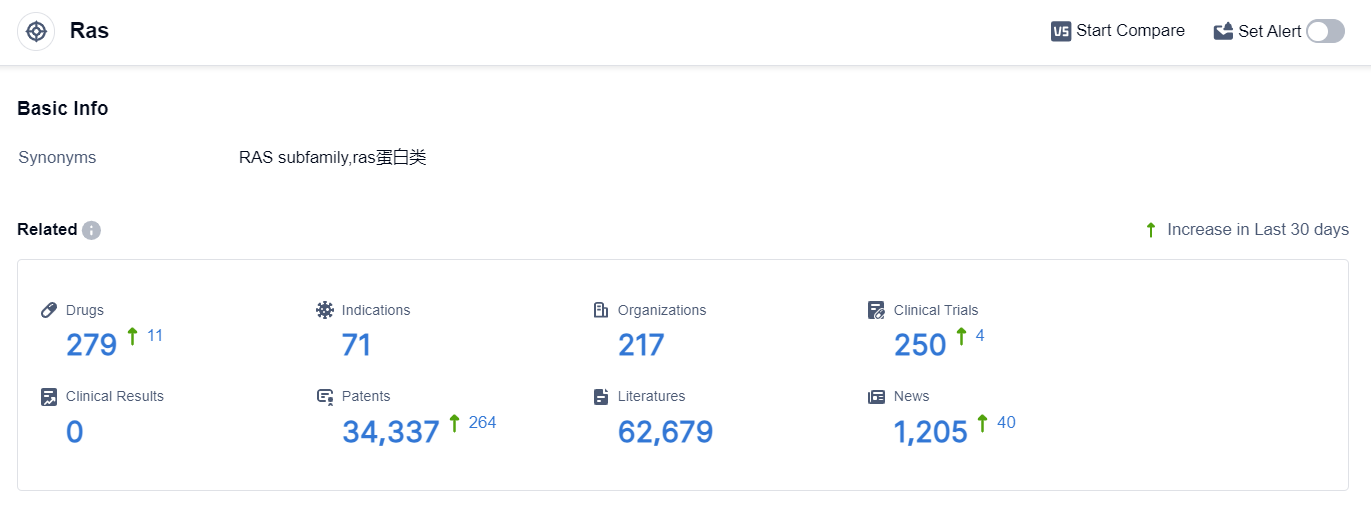
The current competitive landscape of target Ras in the pharmaceutical industry is characterized by the involvement of multiple companies, with Mirati Therapeutics, Inc., Amgen, Inc., and Novartis AG leading the way in terms of R&D progress.
The indications for drugs targeting Ras cover a wide range of cancers, indicating the potential for these therapies in various tumor types. Small molecule drugs are the most rapidly progressing drug type, with intense competition from biosimilars.
The United States, China, and European Union are the key locations driving the development of drugs targeting Ras, with China showing significant progress. Overall, the future development of target Ras in the pharmaceutical industry holds promise for innovative therapies and potential advancements in cancer treatment.
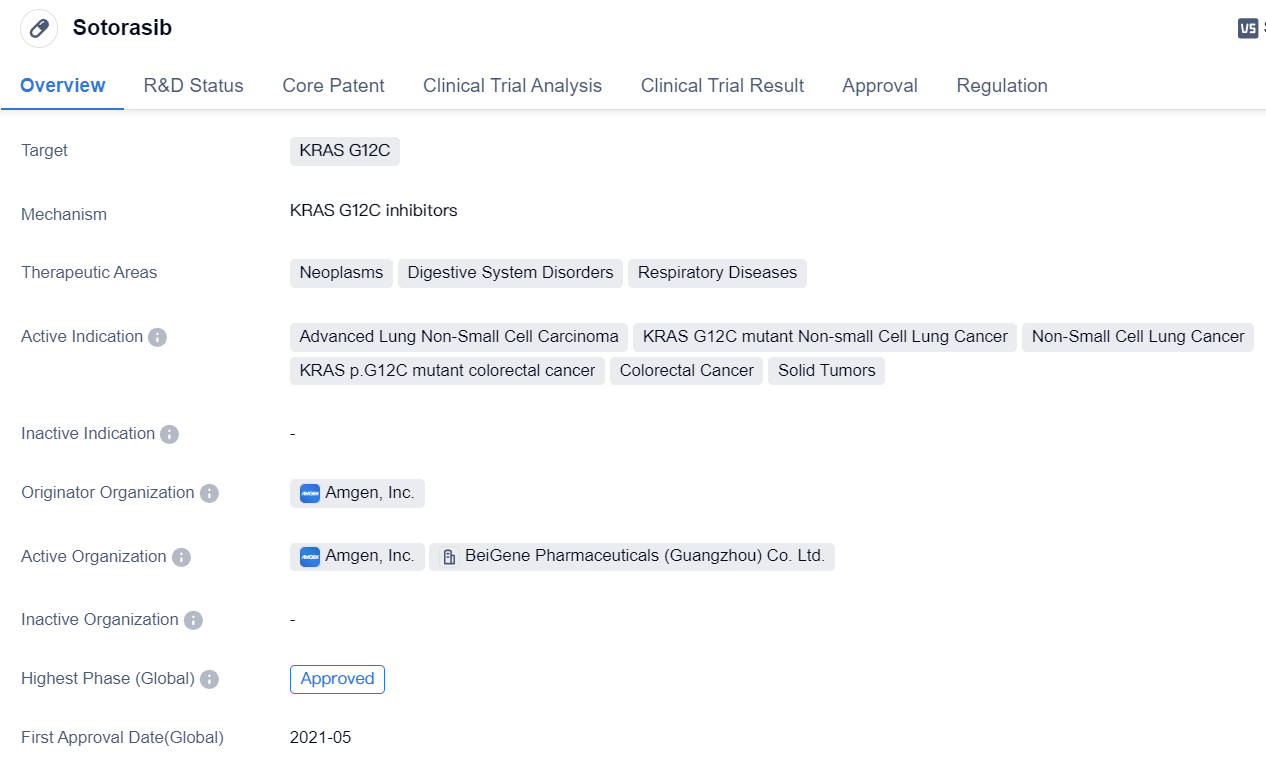
Key drug:Sotorasib
Sotorasib is a small molecule drug developed by Amgen, Inc. It falls under the category of biomedicine and is primarily used to target KRAS G12C, a specific mutation found in various types of cancers. The drug has shown potential therapeutic benefits in the treatment of neoplasms, digestive system disorders, and respiratory diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Sotorasib's active indications include advanced lung non-small cell carcinoma, KRAS G12C mutant non-small cell lung cancer, non-small cell lung cancer, KRAS p.G12C mutant colorectal cancer, colorectal cancer, and solid tumors. These indications highlight the drug's potential in treating a wide range of cancer types, particularly those associated with the KRAS G12C mutation.
The highest phase of development for Sotorasib is approved globally, indicating that it has successfully completed clinical trials and received regulatory approval for commercial use. In China, the drug is currently in phase 3 of development, suggesting that it is undergoing further testing and evaluation in that market.
Sotorasib received its first approval in the United States in May 2021, making it available for use in the treatment of eligible patients. The drug has been granted several regulatory designations, including fast track, breakthrough therapy, accelerated approval, and orphan drug status. These designations signify the potential of Sotorasib to address unmet medical needs and expedite its development and availability for patients.
In summary, Sotorasib is a small molecule drug developed by Amgen, Inc. that targets the KRAS G12C mutation. It has shown promise in treating various types of cancers, including advanced lung non-small cell carcinoma and colorectal cancer. With its recent approval in the United States and ongoing phase 3 development in China, Sotorasib represents a significant advancement in the field of biomedicine for the treatment of specific cancer mutations.
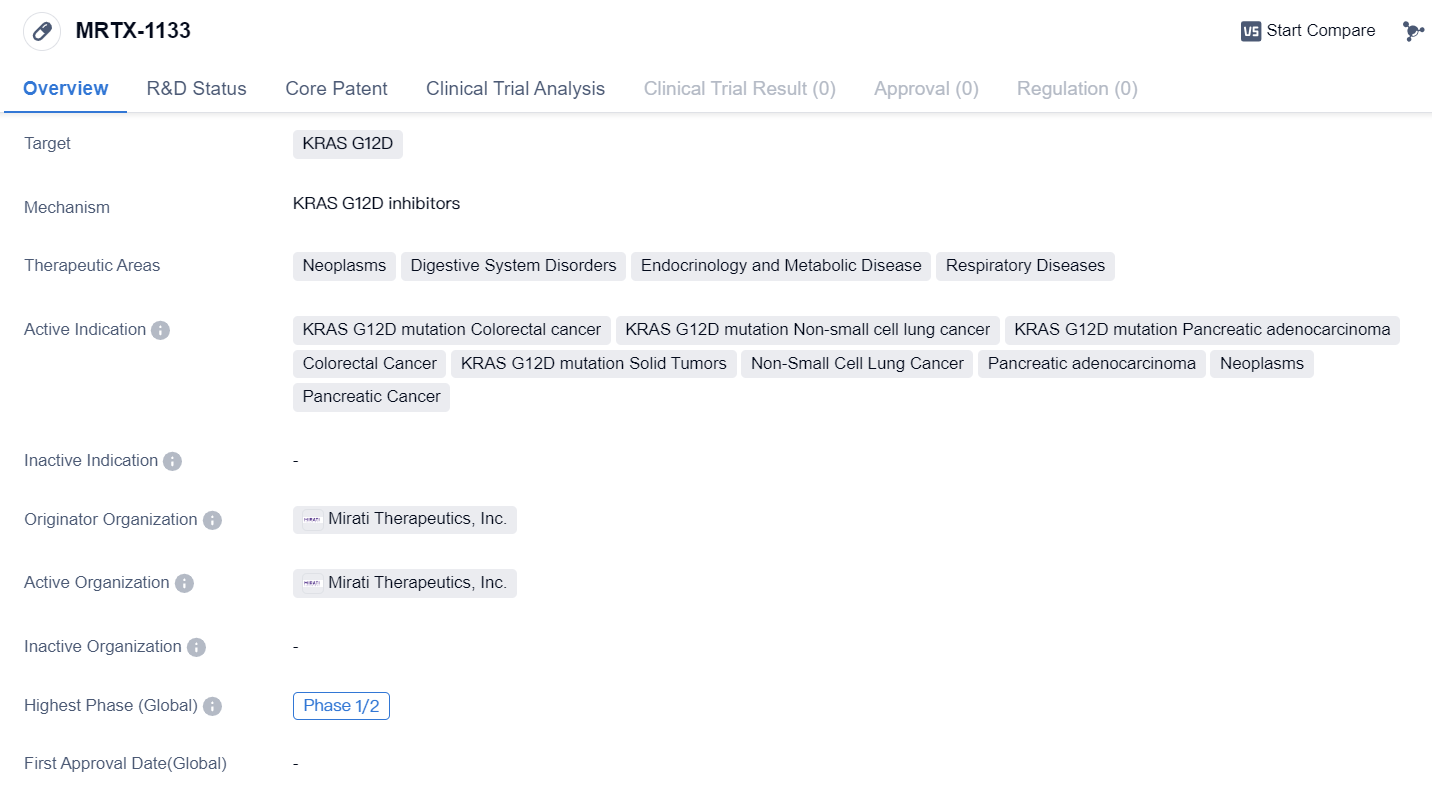
MRTX-1133
MRTX-1133 is a small molecule drug developed by Mirati Therapeutics, Inc. It falls under the category of biomedicine and is primarily designed to target the KRAS G12D mutation. This mutation is associated with various types of cancers, including colorectal cancer, non-small cell lung cancer, and pancreatic adenocarcinoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic areas in which MRTX-1133 is expected to be effective include neoplasms, digestive system disorders, endocrinology and metabolic diseases, and respiratory diseases. These areas encompass a wide range of conditions, highlighting the potential versatility of this drug.
The active indications for MRTX-1133 include colorectal cancer with the KRAS G12D mutation, non-small cell lung cancer with the KRAS G12D mutation, pancreatic adenocarcinoma with the KRAS G12D mutation, colorectal cancer, solid tumors with the KRAS G12D mutation, non-small cell lung cancer, pancreatic adenocarcinoma, neoplasms, and pancreatic cancer. This suggests that MRTX-1133 has the potential to be used in the treatment of various cancers, particularly those associated with the KRAS G12D mutation.
Currently, MRTX-1133 is in Phase 1/2 of development. This indicates that the drug has undergone initial testing in humans and is now being further evaluated for its safety and efficacy. Phase 1/2 trials typically involve a small number of participants and aim to determine the appropriate dosage and potential side effects of the drug.
In summary, MRTX-1133 is a small molecule drug developed by Mirati Therapeutics, Inc. It targets the KRAS G12D mutation and has potential applications in the treatment of various cancers, including colorectal cancer, non-small cell lung cancer, and pancreatic adenocarcinoma. The drug is currently in Phase 1/2 of development, indicating that it is undergoing further evaluation for its safety and effectiveness.