New drugs in the field of diabetes - SGLT1 Inhibitors
SGLT1, or sodium-glucose co-transporter 1, plays a crucial role in the human body by facilitating the absorption of glucose and galactose in the small intestine. It is primarily responsible for the uptake of these sugars from the diet into the bloodstream. SGLT1 is a transmembrane protein located on the apical surface of intestinal epithelial cells, where it actively transports glucose and galactose against their concentration gradient, utilizing the energy derived from sodium ion movement. This process ensures efficient nutrient absorption and helps maintain glucose homeostasis. Understanding the function of SGLT1 is essential for developing therapeutic strategies targeting glucose metabolism and managing conditions like diabetes.
SGLT1 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 25 Sep 2023, there are a total of 18 SGLT1 drugs worldwide, from 23 organizations, covering 16 indications, and conducting 111 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.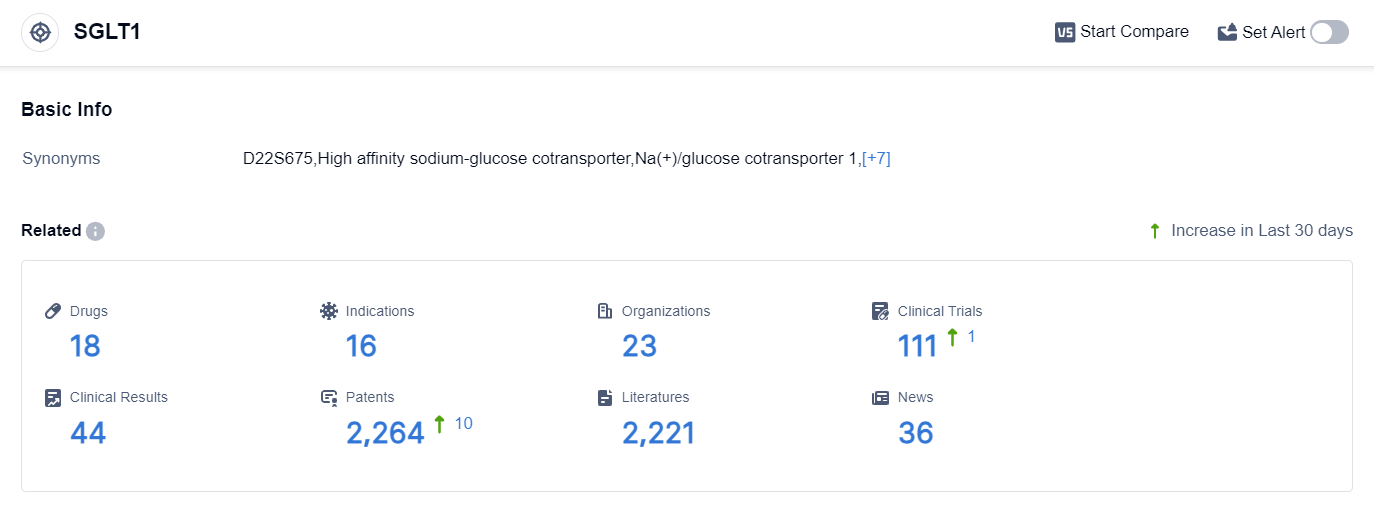
The analysis of the current competitive landscape of target SGLT1 reveals that Lexicon Pharmaceuticals, Inc., Sanofi, Youngene Therapeutics Inc., Ltd., and Ruicheng Xinyusheng Technology Co. Ltd. are the leading companies in terms of R&D progress.
The approved drugs targeting SGLT1 have indications in various diseases such as Heart Failure, Diabetes Mellitus, Chronic Kidney Diseases, and more. Small molecule drugs and Unknown drugs are progressing rapidly, indicating potential therapeutic options.
The development of target SGLT1 is taking place in multiple countries/locations, with the United States, United Kingdom, and European Union being prominent. The future development of target SGLT1 holds promise for the treatment of various diseases and further advancements in pharmaceutical research and development.
Approved for listing SGLT1/2 dual inhibitor: Sotagliflozin
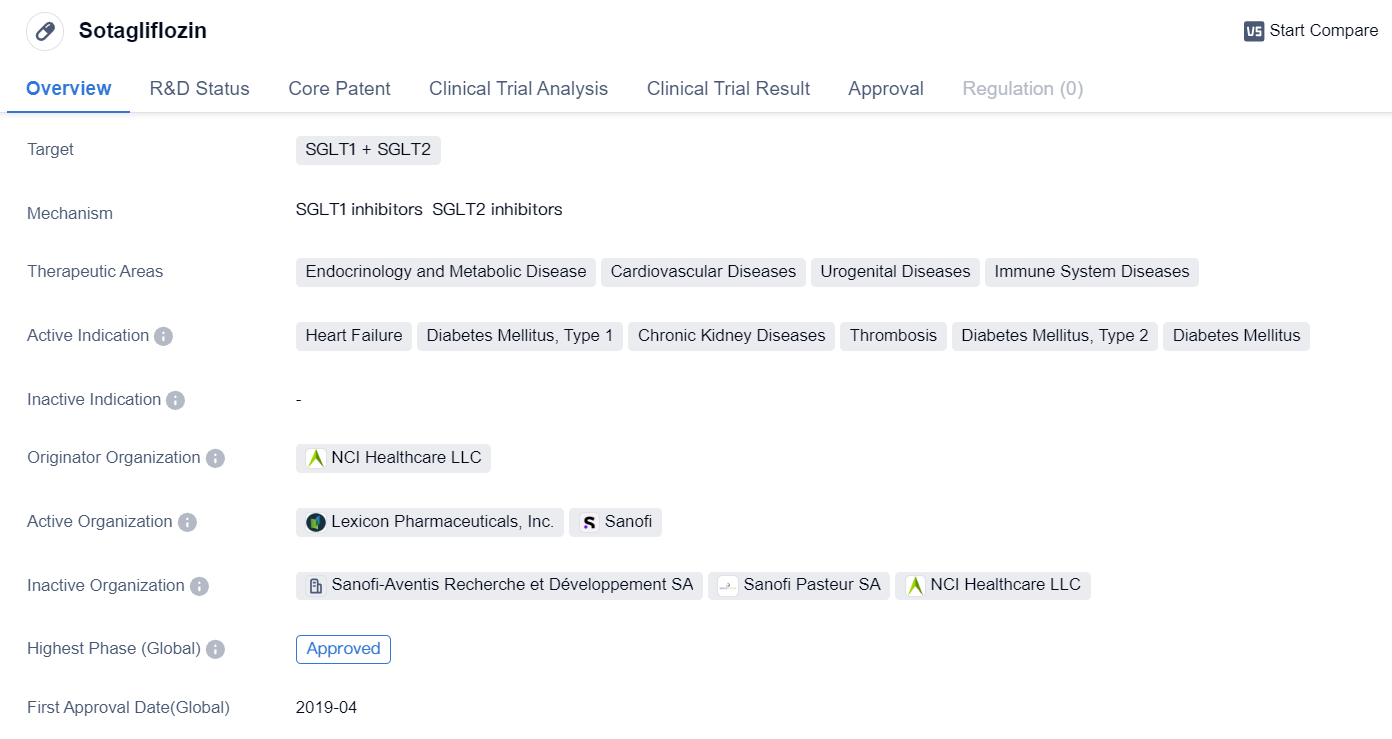
Sotagliflozin is a small molecule drug that targets both SGLT1 and SGLT2. It has shown potential in treating various therapeutic areas including endocrinology and metabolic disease, cardiovascular diseases, urogenital diseases, and immune system diseases. The drug has been indicated for the treatment of heart failure, diabetes mellitus type 1 and type 2, chronic kidney diseases, and thrombosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Sotagliflozin is NCI Healthcare LLC. The drug has reached the highest phase of development, which is approved globally. However, in China, it is currently in Phase 1 of development.
Sotagliflozin received its first approval in April 2019 in the European Union, Iceland, Liechtenstein, and Norway. This indicates that the drug has met the necessary regulatory requirements and has been deemed safe and effective for use in these regions.
The approval of Sotagliflozin in multiple therapeutic areas suggests its potential to address various medical conditions. Its targeting of both SGLT1 and SGLT2 receptors may provide a unique mechanism of action compared to other drugs in the market. By inhibiting these receptors, Sotagliflozin may help regulate glucose levels and improve cardiovascular and renal outcomes in patients with diabetes and heart failure.
As Sotagliflozin progresses through Phase 1 in China, it will undergo further clinical trials and evaluations to assess its safety and efficacy in the Chinese population. If successful, it may eventually receive approval in China as well, expanding its market reach.
In summary, Sotagliflozin is a small molecule drug that targets SGLT1 and SGLT2 receptors. It has shown promise in treating various therapeutic areas and has been approved in the European Union, Iceland, Liechtenstein, and Norway. With its unique mechanism of action and potential benefits in diabetes and heart failure, Sotagliflozin may have a significant impact on the pharmaceutical industry and patient care.
SGLT1 inhibitor entering phase II clinical trials: SY-009
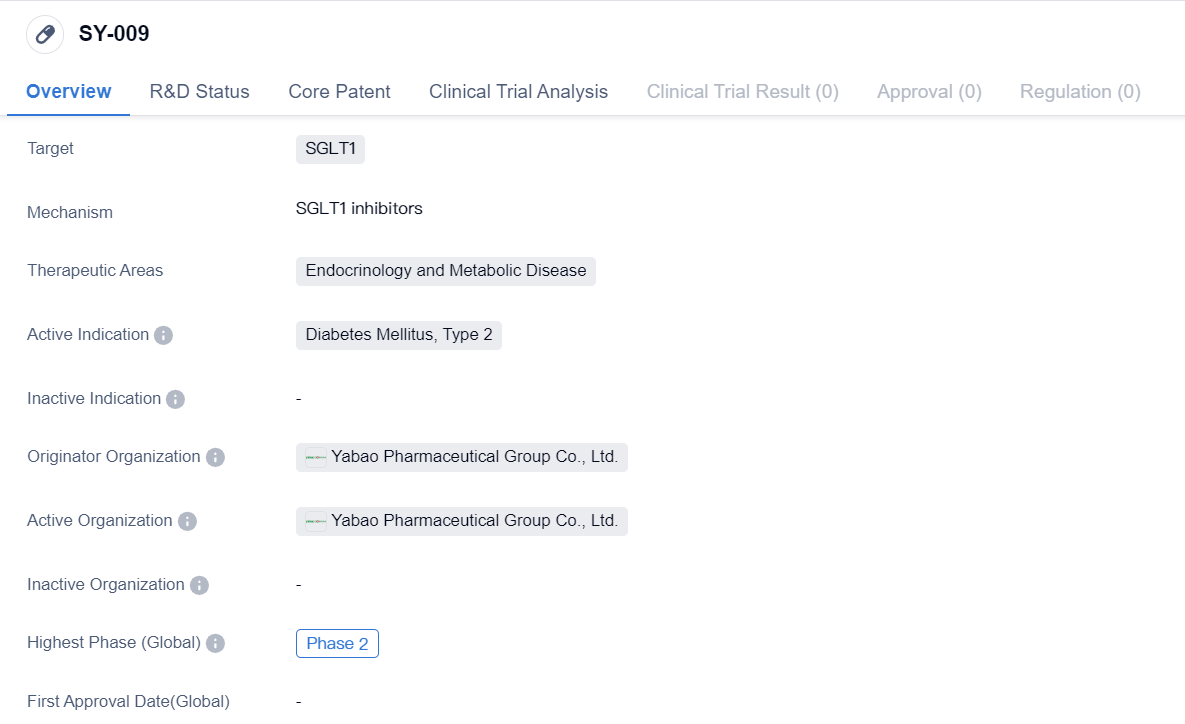
SY-009 is a small molecule drug that targets SGLT1 and is being developed for the treatment of Diabetes Mellitus, Type 2. It falls under the therapeutic areas of Endocrinology and Metabolic Disease. The drug is being developed by Yabao Pharmaceutical Group Co., Ltd., an originator organization in the pharmaceutical industry.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Currently, SY-009 has reached Phase 2 of development, both globally and in China. Phase 2 is a critical stage in drug development where the drug's safety and efficacy are further evaluated in a larger group of patients. This phase helps determine the optimal dosage, potential side effects, and overall effectiveness of the drug.
The specific target of SY-009, SGLT1, is a protein involved in glucose transport in the body. By targeting SGLT1, the drug aims to regulate glucose levels in patients with Type 2 Diabetes Mellitus. This therapeutic approach aligns with the drug's intended use in the treatment of this specific disease.
Endocrinology and Metabolic Disease is a broad therapeutic area that encompasses various conditions related to hormone imbalances and metabolic disorders. Diabetes Mellitus, Type 2, is a prevalent metabolic disease characterized by high blood sugar levels due to insulin resistance or insufficient insulin production. The development of SY-009 in this therapeutic area indicates a focus on addressing the unmet medical needs of patients with Type 2 Diabetes Mellitus.
Yabao Pharmaceutical Group Co., Ltd. is the originator organization responsible for the development of SY-009. As an originator, they are the primary developers and hold the intellectual property rights for the drug. Yabao Pharmaceutical Group Co., Ltd. is likely to have invested significant resources in the research and development of SY-009, with the aim of bringing a novel treatment option to the market for patients with Type 2 Diabetes Mellitus.
In summary, SY-009 is a small molecule drug being developed by Yabao Pharmaceutical Group Co., Ltd. It targets SGLT1 and is intended for the treatment of Diabetes Mellitus, Type 2. The drug has reached Phase 2 of development, indicating progress in evaluating its safety and efficacy. With a focus on the therapeutic area of Endocrinology and Metabolic Disease, SY-009 aims to address the unmet medical needs of patients with Type 2 Diabetes Mellitus.