Alvotech reveals Japan’s authorization of AVT04 (ustekinumab), a biosimilar counterpart to Stelara®
Alvotech, an international biotech firm known for creating and producing biosimilar drugs for a global patient base, revealed that its distribution associate in Japan, Fuji Pharma Co., Ltd., has obtained commercialisation authorization for AVT04 (ustekinumab), a biosimilar counterpart to Stelara, this approval comes from the Health, Labor and Welfare Ministry of Japan.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
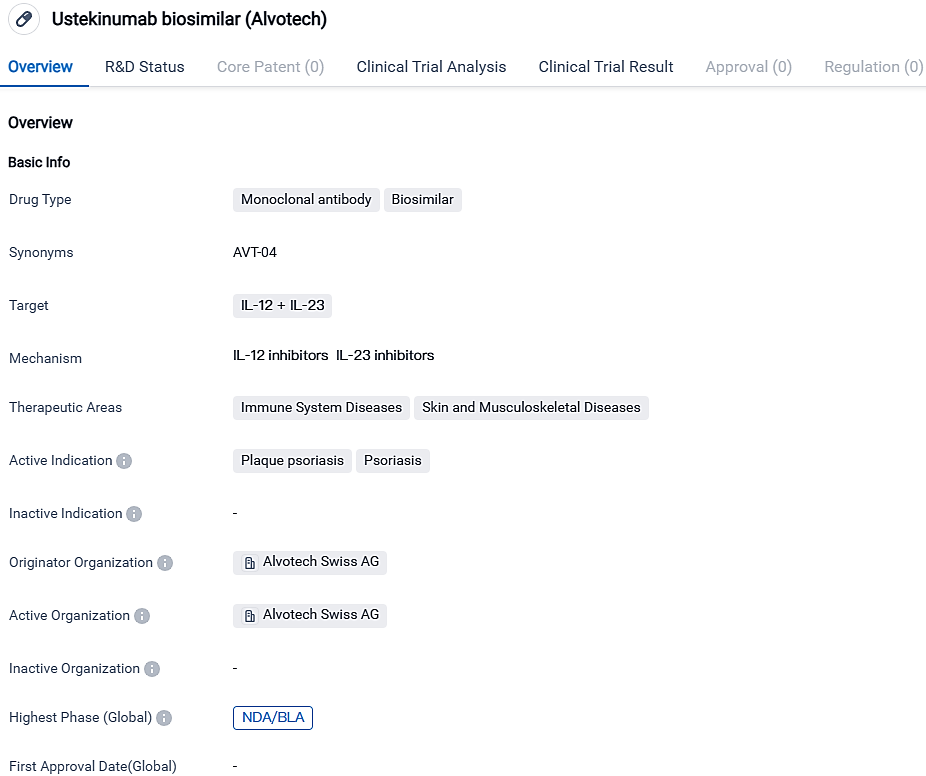
From the data accessible to the public, AVT04 stands as the initial biosimilar of Stelara given the green light for distribution in the worldwide markets. Stelara's international sales topped $10 billion during the year leading up to June 30, 2023, as evidenced by the manufacturer's public documents, positioning it among the globe's top revenue-generating biologics.
Robert Wessman, chairman and CEO of Alvotech, expressed his delight at the company gaining the authorization to market AVT04 in Japan, representing the company's second biosimilar in the market. Noting that both Alvotech and Fuji share a common goal of expanding patient access to indispensable biologic medicines, he expressed confidence in their robust partnership serving the rising demand for biosimilars in Japan, based on their robust partnership.
AVT04 was created using a Sp2/0 host cell line and Alvotech is responsible for its production via a continuous perfusion process. The Sp2/0 host cell line enables the molecule to sialylation with greater efficiency comparably to Chinese hamster ovary cells and it is the identical type of host cell line used in the production of Stelara.
This represents the foremost biosimilar approved under Alvotech's business partnership with Fuji. It is part of an initiative that also involves six additional biosimilar candidates, slated for development and production by Alvotech and marketing by Fuji in Japan. This collaboration with Fuji was first made public in November of 2018.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
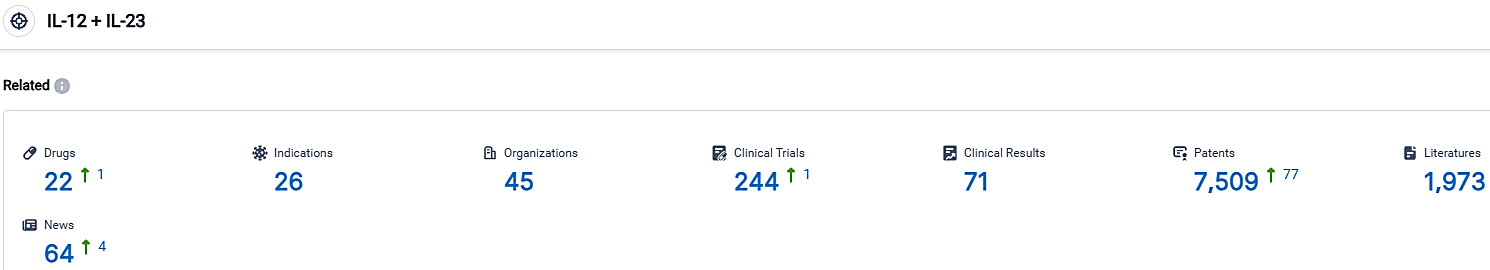
According to the data provided by the Synapse Database, As of October 1, 2023, there are 22 investigational drugs for the IL-12 and IL-23 target, including 26 indications,45 R&D institutions involved, with related clinical trials reaching 244,and as many as 7509 patents.
AVT04 is a biosimilar prospect of Stelara (ustekinumab), and it's a monoclonal antibody. It is designed to attach to two specific cytokines, namely IL-12 and IL-23, which take part in immune and inflammatory reactions. Currently, AVT04 has only been authorized for use in Japan and remains under the investigative phase. It's being reviewed in other key international markets.