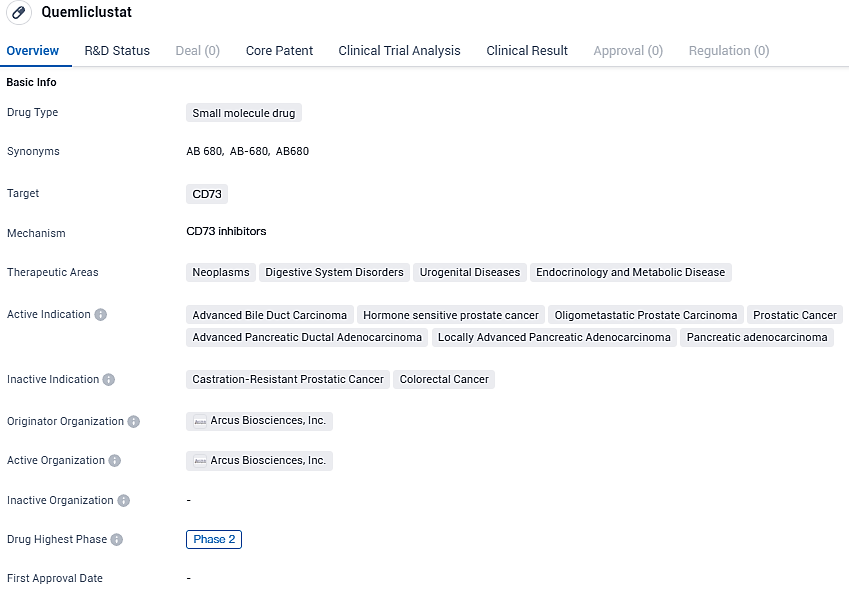
Preliminary results from a Phase 1b trial indicate that Quemliclustat may improve survival in untreated advanced pancreatic cancer patients
Arcus Biosciences, Inc., Incorporated has released encouraging data regarding overall patient survival from their collaborative Phase 1b trial, ARC-8, which is in partnership with Gilead Sciences. The research conducted in ARC-8 examines the efficacy of the novel small molecule CD73 inhibitor, quemliclustat, in combination with standard chemotherapy treatments. This regimen may also include zimberelimab, a new anti-PD-1 antibody under investigation, for the treatment of individuals diagnosed with metastatic pancreatic ductal adenocarcinoma who have not undergone any previous therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"A therapeutic strategy incorporating quemliclustat has been shown to significantly extend life expectancy beyond the typical outcomes seen in mPDAC patients who undergo only traditional chemotherapy, which has been the mainstay for over three decades," reported Zev A. Wainberg, MD, MSc, who is the Co-Lead of the GI Oncology Program at the University of California, Los Angeles and one of the chief researchers for the ARC-8 study.
"Pancreatic cancer cells are known to overexpress CD73, and the preliminary signs that blocking CD73 with a small molecule can enhance patient prognoses with mPDAC are promising," continued Wainberg. "This appears to be achievable with coinciding standard chemotherapy and does not seem to significantly increase treatment-related side effects, according to our early comparisons with past outcomes from chemotherapy treatments alone."
The forthcoming presentation will encompass outcomes from all the treatment-naive mPDAC patients enrolled in the ARC-8 trial. These patients were administered 100mg of quemliclustat in combination with chemotherapy, with some additionally receiving zimberelimab. These participants were part of the initial dose-finding, dose-expansion, and randomized study groups. The cut-off point for this collected data was June 19, 2023. When compared to historical control data, which indicates a median overall survival (mOS) of roughly nine months with chemotherapy alone, the quemliclustat regimens showed numerically enhanced survival figures.
A post-hoc analysis has also been carried out by the Medidata AI team, a segment of Medidata Solutions, under Dassault Systèmes. This involved the creation of a Synthetic Control Arm representing patients who had received the gemcitabine/nab-paclitaxel duo in Phase 2 and 3 trials as their initial line of attack against advanced pancreatic cancer.
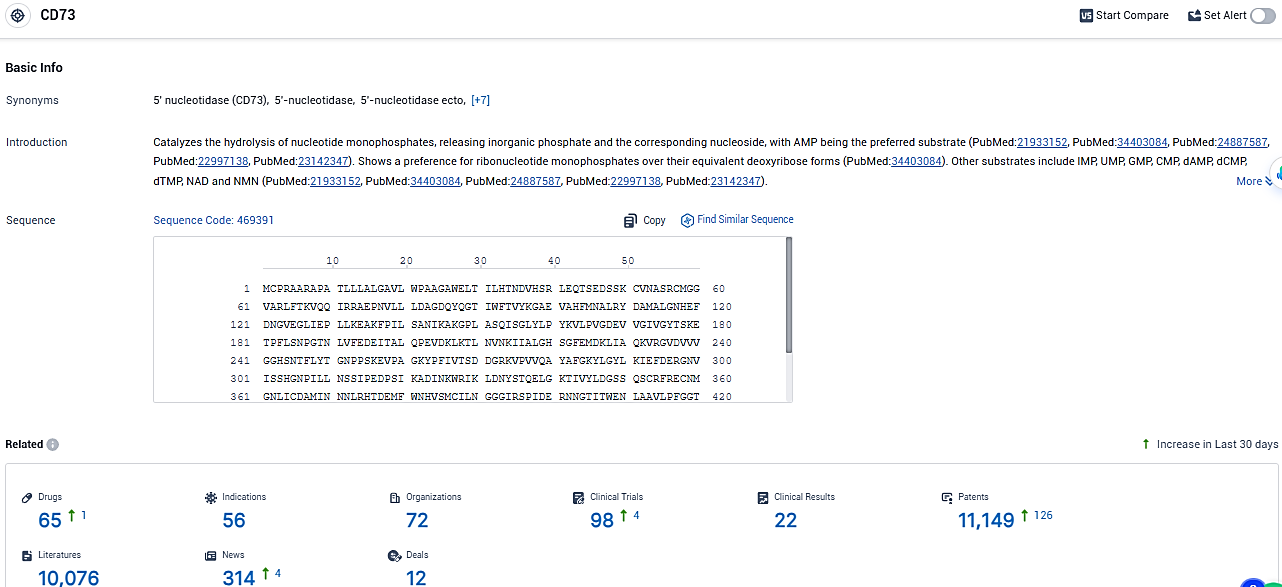
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 23, 2024, there are 65 investigational drugs for the CD73 target, including 56 indications, 72 R&D institutions involved, with related clinical trials reaching 98, and as many as 11149 patents.
Quemliclustat, a small molecule drug targeting CD73, shows promise in various therapeutic areas, particularly in the treatment of advanced bile duct carcinoma, hormone-sensitive prostate cancer, oligometastatic prostate carcinoma, and advanced stages of pancreatic ductal adenocarcinoma. As it progresses through clinical trials, further research will be needed to determine its safety and efficacy in these indications.