Vertex Announces FDA Approval for CASGEVY™ (exagamglogene autotemcel) as Treatment for Blood Transfusion-Dependent Beta Thalassemia
Vertex Pharmaceuticals Incorporated disclosed that it has received approval from the U.S. Food and Drug Administration for its innovative therapy, CASGEVY™ (exagamglogene autotemcel [exa-cel]), This advanced CRISPR/Cas9 gene-editing cellular treatment has been sanctioned for use in individuals aged 12 and above who require the treatment of transfusion-dependent beta thalassemia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Following the groundbreaking endorsement of CASGEVY by the FDA for addressing sickle cell disease, we are thrilled to announce the advanced authorization for TDT, well before the anticipated PDUFA deadline," expressed Dr. Reshma Kewalramani, CEO and President of Vertex. "Individuals with TDT are in need of innovative treatments that may offer a cure. Our team is eager to make CASGEVY available to the patients who are eagerly awaiting it."
To administer CASGEVY proficiently, expertise in stem cell transplantation is necessary. Consequently, Vertex is collaborating with reputable medical facilities to set up a series of certified, autonomous treatment centers across the United States to provide access to CASGEVY for those who qualify. Currently, all nine of the established Authorized Treatment Centers (ATCs) in the United States are equipped to administer CASGEVY to qualified individuals with TDT or sickle cell disease. The network will expand shortly, with additional centers being prepared for operation. A comprehensive roster of these ATCs is available on the website CASGEVY.com.
TDT represents a grave and potentially fatal genetic condition. Individuals with TDT tend to have lower health-related quality of life indices than the general populace, and the lifelong medical expenses associated with managing TDT in the United States are projected to lie between $5 million and $5.7 million. TDT management typically involves regular blood transfusions and continuous iron chelation therapy. Anemia in TDT patients often results in symptoms like fatigue and breathing difficulties, while infants might face growth failure, yellowing of the skin, and nutritional issues. Additional health complications linked to TDT include enlargement of spleen, liver, and/or heart, bone deformities, and stunted sexual development.
The condition necessitates ongoing management and places a substantial burden on the healthcare system, often leading to a poorer quality of life and shorter lifespan, as well as lost income and productivity. In the United States, the median lifespan for those living with TDT stands at 37 years. While stem cell transplantation from a matched donor may offer a cure, it remains an option for only a select number of TDT patients due to the scarcity of suitable donors.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
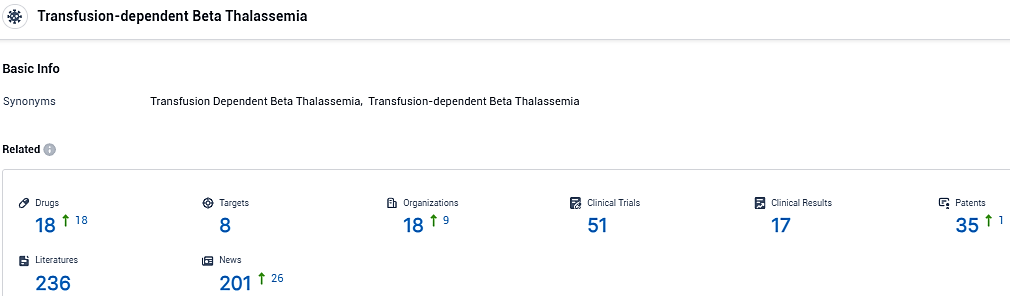
According to the data provided by the Synapse Database, As of January 23, 2024, there are 18 investigational drugs for the transfusion-dependent beta thalassemia, including 8 targets, 18 R&D institutions involved, with related clinical trials reaching 51, and as many as 17 patents.
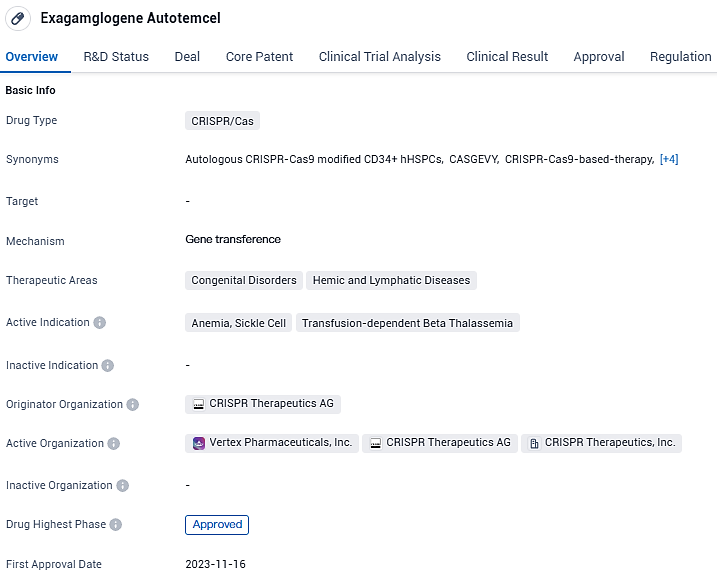
Exagamglogene Autotemcel has been approved for the treatment of congenital disorders and hemic and lymphatic diseases, specifically anemia, sickle cell disease, and transfusion-dependent beta thalassemia. The drug has received regulatory approvals and designations, indicating its potential significance in the field of biomedicine. Its first approval occurred in the United Kingdom in November 2023.