Adicet Initiates Phase 1 Trial Recruitment for ADI-270 in Advanced Renal Cell Carcinoma
Adicet Bio, Inc. (Nasdaq: ACET), a biotechnology firm focused on clinical development, specializes in identifying and creating allogeneic gamma delta T cell treatments targeting autoimmune disorders and cancer. The company has officially begun the enrollment process for its Phase 1 clinical trial assessing ADI-270 in individuals diagnosed with metastatic or advanced clear cell renal cell carcinoma (ccRCC).
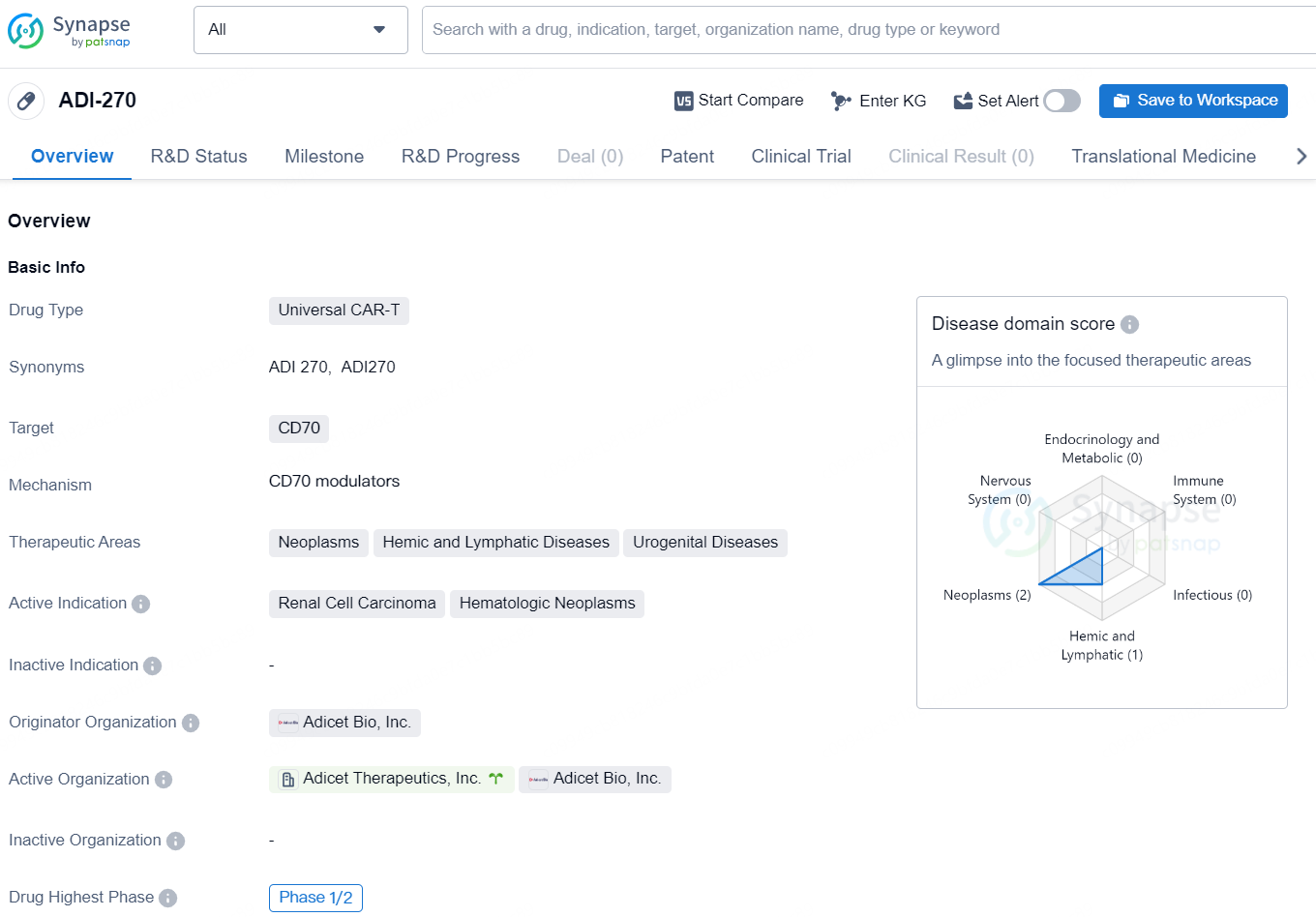
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
“Solid tumors represent a significant area of unmet medical needs within oncology and have not yet experienced the advancements seen with CAR T cell therapies for hematologic cancers. New findings from ADI-270, our engineered allogeneic ‘off the shelf’ gamma delta 1 CAR T cell therapy targeting CD70-positive tumors, indicate its promise in addressing this issue,” stated Chen Schor, President and Chief Executive Officer. “During the recent ASGCT conference, we shared preclinical findings showing that ADI-270 exhibited considerable tumor infiltration, resilience against the immunosuppressive tumor microenvironment, and strong activity through CAR and innate-mediated targeting. This underscores its potential use in solid tumor treatment. We eagerly anticipate the enrollment of patients and expect to release initial clinical data from the trial in the first half of 2025.”
The Phase 1 multicenter, open-label clinical study aims to evaluate ADI-270 as a standalone treatment in adults with relapsed or refractory ccRCC. After lymphodepletion, eligible patients can receive a single dose of ADI-270, beginning with a starting dose of 3E8 CAR+ cells. Depending on the fulfillment of protocol-defined criteria, trial participants may qualify for an additional dose of ADI-270. The trial's dose escalation and dose expansion phases will assess safety, tolerability, pharmacokinetics, and antitumor efficacy as measured by overall response rate, duration of response, and disease control rate.
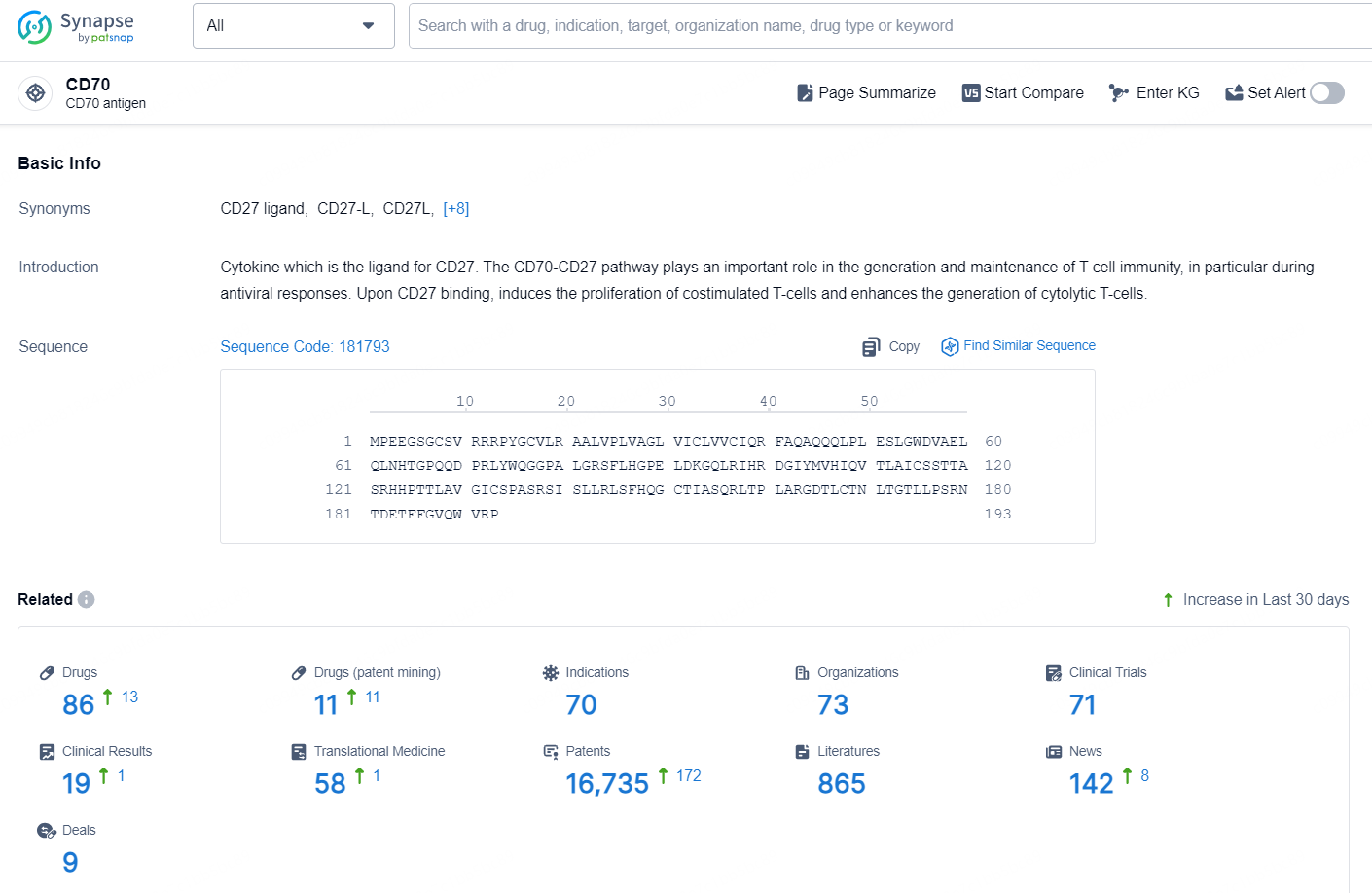
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 25, 2024, there are 86 investigational drugs for the CD70 target, including 70 indications, 73 R&D institutions involved, with related clinical trials reaching 71, and as many as 16735 patents.
ADI-270 is a Universal CAR-T drug developed by Adicet Bio, Inc. The drug targets CD70 and is primarily intended for the treatment of Neoplasms, Hemic and Lymphatic Diseases, and Urogenital Diseases. The active indications for ADI-270 include Renal Cell Carcinoma and Hematologic Neoplasms.