Unleashing the Power of Vadadustat: A Comprehensive Review on R&D Breakthroughs
Vadadustat's R&D Progress
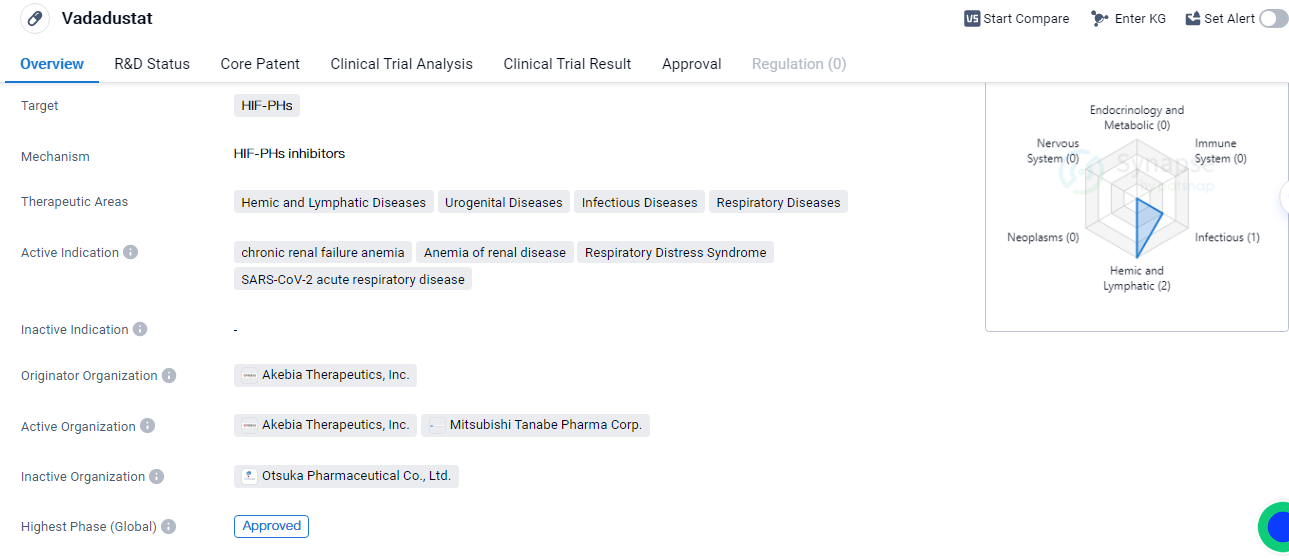
Vadadustat is a small molecule drug that falls under the category of biomedicine. It targets HIF-PHs, which are enzymes involved in the regulation of hypoxia-inducible factors. The drug has shown potential in treating various therapeutic areas, including hemic and lymphatic diseases, urogenital diseases, infectious diseases, and respiratory diseases.
One of the active indications for Vadadustat is chronic renal failure anemia, a condition characterized by a decrease in red blood cell production due to kidney dysfunction. It is also indicated for anemia of renal disease, which is commonly seen in patients with chronic kidney disease. Additionally, Vadadustat has shown promise in treating respiratory distress syndrome and SARS-CoV-2 acute respiratory disease.
Vadadustat was developed by Akebia Therapeutics, Inc., a pharmaceutical company specializing in the development of innovative therapies. The drug has reached the highest phase of development, with global approval. However, in China, it is currently in phase 2 clinical trials.
The first approval of Vadadustat took place in June 2020 in Japan, which suggests that Vadadustat has undergone rigorous testing and evaluation, demonstrating its potential benefits in addressing the specific medical conditions it is indicated for.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Vadadustat: HIF-PHs inhibitors
HIF-PHs inhibitors are a type of drugs that target and inhibit the activity of hypoxia-inducible factor prolyl hydroxylases (HIF-PHs). HIF-PHs are enzymes that play a crucial role in the regulation of the hypoxia-inducible factor (HIF) pathway.
From a biomedical perspective, the HIF pathway is a cellular signaling pathway that helps the body respond to low oxygen levels (hypoxia). When oxygen levels are low, HIF-PHs hydroxylate specific proline residues on HIF proteins, leading to their degradation. This prevents the activation of HIF and the subsequent expression of genes involved in adapting to hypoxic conditions.
HIF-PHs inhibitors work by blocking the activity of HIF-PHs, thereby preventing the degradation of HIF proteins. This allows HIF proteins to accumulate and activate the expression of genes involved in various cellular processes, including angiogenesis (formation of new blood vessels), erythropoiesis (production of red blood cells), and glucose metabolism.
In a clinical context, HIF-PHs inhibitors have shown promise as potential therapeutic agents for various conditions related to inadequate oxygen supply, such as anemia, ischemic diseases and certain types of cancers. By activating the HIF pathway, these inhibitors can help improve oxygen delivery to tissues and promote adaptive responses to hypoxia. However, further research and clinical trials are needed to determine their safety and efficacy.
Drug Target R&D Trends for Vadadustat
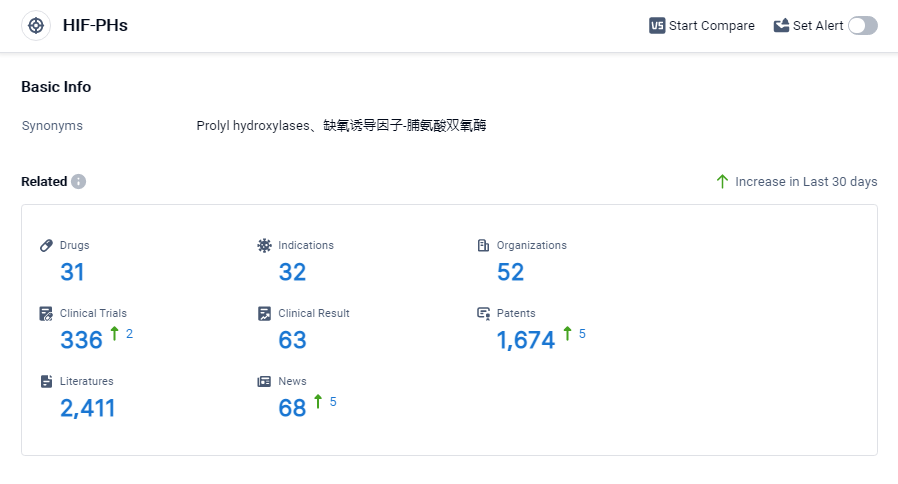
According to Patsnap Synapse, as of 3 Sep 2023, there are a total of 31 HIF-PHs drugs worldwide, from 52 organizations, covering 32 indications, and conducting 336 clinical trials.
Based on the analysis, Akebia Therapeutics, Inc., FibroGen, Inc., and Zydus Lifesciences Ltd. are the companies with the highest stage of development under the target HIF-PHs. Drugs targeting HIF-PHs have been approved for indications such as anemia of renal disease, chronic renal failure anemia, and anemia in chronic kidney disease. Small molecule drugs are progressing rapidly. Japan, China, and the United States are the leading countries in terms of drug development for target HIF-PHs. Overall, the current competitive landscape suggests significant progress in the development of drugs targeting HIF-PHs, with potential for future advancements in the field.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Vadadustat is a small molecule drug developed by Akebia Therapeutics, Inc. It targets HIF-PHs and has shown potential in treating various therapeutic areas, including hemic and lymphatic diseases, urogenital diseases, infectious diseases, and respiratory diseases. Its active indications include chronic renal failure anemia, anemia of renal disease, respiratory distress syndrome, and SARS-CoV-2 acute respiratory disease. The drug was first approved in Japan in June 2020, while it has received global approval, it is currently in phase 2 clinical trials in China.