Advances in Clinical Research on Acetylcholinesterase Inhibitor
Acetylcholinesterase (AChE) is a key enzyme in biological nerve conduction, which can degrade acetylcholine at cholinergic synapses and terminate the excitation of neurotransmitters on the post-synaptic membrane, ensuring the normal transmission of nerve signals within the organism. Acetylcholinesterase participates in cell development and maturation, promoting neuron development and nerve regeneration.
AChE is responsible for breaking down the neurotransmitter acetylcholine, which is involved in various physiological processes such as muscle contraction, cognition, and memory. AChE ensures the proper functioning of the cholinergic system by rapidly hydrolyzing acetylcholine, preventing its accumulation and maintaining a delicate balance. Dysfunction or inhibition of AChE can lead to an excess of acetylcholine, resulting in various neurological disorders like Alzheimer's disease and myasthenia gravis. Understanding the role of AChE is vital for developing therapeutic strategies to regulate cholinergic activity and treat associated conditions.
Acetylcholinesterase Competitive Landscape
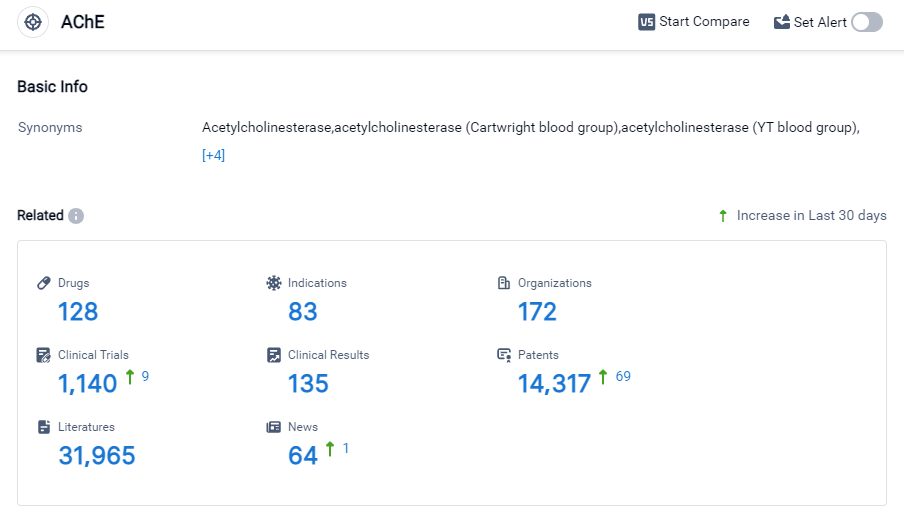
According to Patsnap Synapse, as of 27 Sep 2023, there are a total of 128 AChE drugs worldwide, from 172 organizations, covering 83 indications, and conducting 1140 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target AChE reveals that AbbVie, Inc., Shanghai Pharmaceuticals Holding Co., Ltd., Pfizer Inc., Zeria Pharmaceutical Co., Ltd., and Eisai Co., Ltd. are the companies growing fastest under this target. These companies have made significant progress in R&D, with multiple approved drugs for the target AChE. The indications for these drugs cover a wide range of neurological and gastrointestinal disorders, indicating the potential therapeutic applications of drugs targeting AChE. Small molecule drugs are the primary focus of research and development for this target, with a significant number of approved and preclinical drugs. The countries/locations developing fastest under the target AChE are China, the United States, Japan, and the European Union. China, in particular, has made significant progress with a high number of approved drugs. Overall, the competitive landscape for the target AChE is dynamic, with multiple companies and countries actively involved in R&D and the development of drugs for various indications. The future development of target AChE holds promise for the treatment of neurological and gastrointestinal disorders.
The latest approved Acetylcholinesterase inhibitor: Neostigmine Methylsulfate/Glycopyrrolate
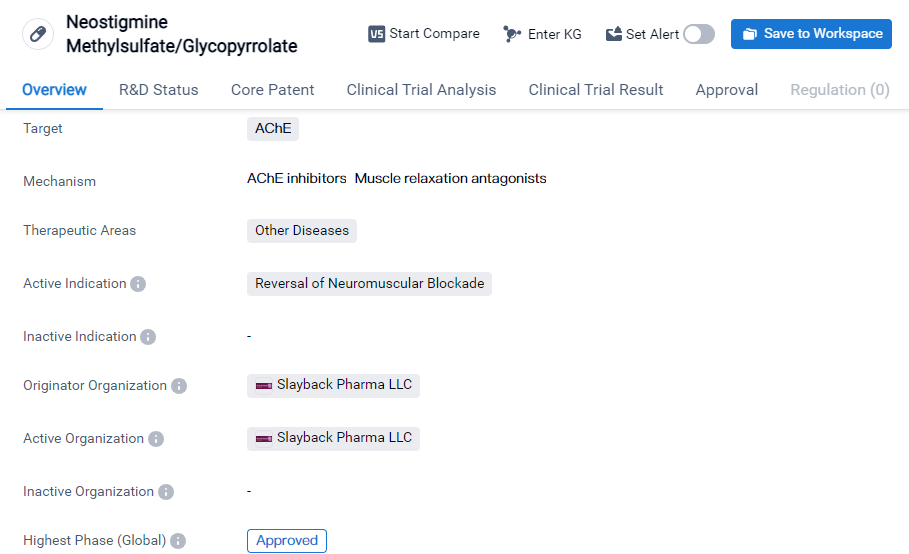
Neostigmine Methylsulfate/Glycopyrrolate is a small molecule drug that targets AChE (Acetylcholinesterase). It falls under the therapeutic area of other diseases and is specifically indicated for the reversal of neuromuscular blockade. The drug has been developed by Slayback Pharma LLC, an originator organization in the pharmaceutical industry.
As of the highest phase, Neostigmine Methylsulfate/Glycopyrrolate has received approval. This means that it has successfully completed the necessary clinical trials and regulatory processes to be deemed safe and effective for use. The drug has obtained global approval, indicating its potential for international distribution and availability.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The first approval of Neostigmine Methylsulfate/Glycopyrrolate took place in February 2023, with the United States being the country/location where it was granted. This suggests that the drug has met the stringent requirements set by the U.S. regulatory authorities, such as the Food and Drug Administration (FDA), for its commercialization and use within the country.
Neostigmine Methylsulfate/Glycopyrrolate's primary function is to reverse neuromuscular blockade. Neuromuscular blockade refers to the temporary paralysis of muscles, often induced during surgical procedures or in intensive care settings. By targeting AChE, the drug aims to restore normal muscle function by inhibiting the breakdown of acetylcholine, a neurotransmitter responsible for muscle contraction.
The approval of Neostigmine Methylsulfate/Glycopyrrolate marks a significant milestone in the field of biomedicine. It provides healthcare professionals with a valuable tool for managing and reversing neuromuscular blockade, ultimately improving patient outcomes and safety during surgical procedures and critical care situations.
In conclusion, Neostigmine Methylsulfate/Glycopyrrolate is a small molecule drug developed by Slayback Pharma LLC. It has received global approval and is indicated for the reversal of neuromuscular blockade. The drug's first approval occurred in the United States in February 2023. Its successful development and approval offer promising prospects for addressing the challenges associated with neuromuscular blockade in various medical settings.