AI Empowers Small Nucleic Acid Drugs: New Strategies from Target Mining to Chemical Modification
The application of Artificial Intelligence (AI) in the development of small nucleic acid drugs is becoming a significant force in driving the progress of this field. AI technologies, through means such as machine learning (ML) and deep learning (DL), are playing an increasingly important role in various stages of drug development, including target discovery, compound screening, and drug design.
In the development of small nucleic acid drugs, the application of AI can significantly shorten the time required for drug development. For instance, AI technology can predict the three-dimensional structure of small nucleic acid drugs, which is crucial for understanding how the drugs interact with their targets. AI can also rapidly screen potential drug candidates from a vast array of compounds, optimize the biological effects of drug molecules, and even predict unexpected activities to repurpose drugs. These advancements provide new strategies and tools for drug development, especially in the field of small nucleic acid drugs, where AI applications help to enhance the targeting and efficacy of the drugs.
The Role of AI in Drug Target Discovery
The role of AI in drug target discovery is becoming increasingly prominent. By analyzing vast amounts of biomedical data, AI identifies disease-related genes and proteins, thereby accelerating the process of drug target identification. The application of AI technology can significantly improve the efficiency and accuracy of target discovery and reduce the early failure rate in drug development.
Several databases and software play a crucial role in AI-enabled drug target discovery. Comprehensive drug databases provide detailed information about drugs, including their chemical structures, pharmacological actions, drug activity data, and target information. This information can be used for training and validating AI models and is essential for the application of AI in drug target discovery.
In addition to databases and software, AI plays a role in drug target discovery through the application of deep learning models. These models are capable of processing complex biological networks and multi-omics data to identify potential drug targets. For instance, decision tree algorithms, a type of supervised classification algorithm, can categorize gene-phenotype associations by selecting significant network topological features, thereby identifying cancer-related genes. Deep learning models, such as Convolutional Neural Networks (CNNs) and Recurrent Neural Networks (RNNs), can learn patterns from large-scale biomedical data to predict drug targets. The use of AI in drug target discovery has been revolutionized by these advanced techniques, which not only enhance the efficiency and accuracy of target identification but also open up new avenues for drug development.
The Role of AI in Small Nucleic Acid Drug Screening
The role of AI in small nucleic acid drug screening is multifaceted. It analyzes vast amounts of chemical and biomedical data to predict the biological activity and drug-like properties of compounds, thereby accelerating the discovery of drug candidates. AI technology can predict the pharmacological activity, toxicity, absorption, distribution, metabolism, excretion, and toxicity (ADMET) characteristics of compounds, which are crucial for assessing the potential of compounds as drug candidates.
AI, by analyzing extensive biomedical data, can predict the activity and drug-like properties of small nucleic acid drugs, thus accelerating the discovery of potential drug candidates. The application of AI technology not only improves screening efficiency but also helps to optimize drug design, predict the three-dimensional structure of drugs, and their interactions with targets. This is vital for enhancing the stability and delivery efficiency of drugs. Furthermore, AI technology plays a role in the design of drug delivery systems. By predicting the best delivery pathways and carriers, AI helps to improve the efficacy and safety of small nucleic acid drugs. The comprehensive application of AI technology is changing the landscape of small nucleic acid drug development, making it more precise and efficient.
The integration of AI in the discovery and screening of small nucleic acid drugs has significantly accelerated the process and provided robust support for future drug development, indicating the immense potential and application prospects of AI in the pharmaceutical field. With continuous technological advancements, it is anticipated that AI will play an increasingly critical role in the development of small nucleic acid drugs.
The role of AI in small nucleic acid drug design
The role of AI in the design of small nucleic acid drugs is becoming increasingly prominent. AI, by analyzing vast amounts of biomedical data, can predict the activity and pharmacological properties of small nucleic acid drugs, thereby accelerating the discovery of drug candidates. AI technologies are capable of predicting the three-dimensional structures of these drugs, which is crucial for understanding how they interact with their targets. Moreover, AI can rapidly screen a large number of compounds to identify potential drug candidates, optimize the biological effects of drug molecules, and even repurpose drugs by predicting unexpected activities. These advancements provide new strategies and tools for drug development, especially in the field of small nucleic acid drugs, where AI applications help to enhance the targeting and efficacy of drugs.
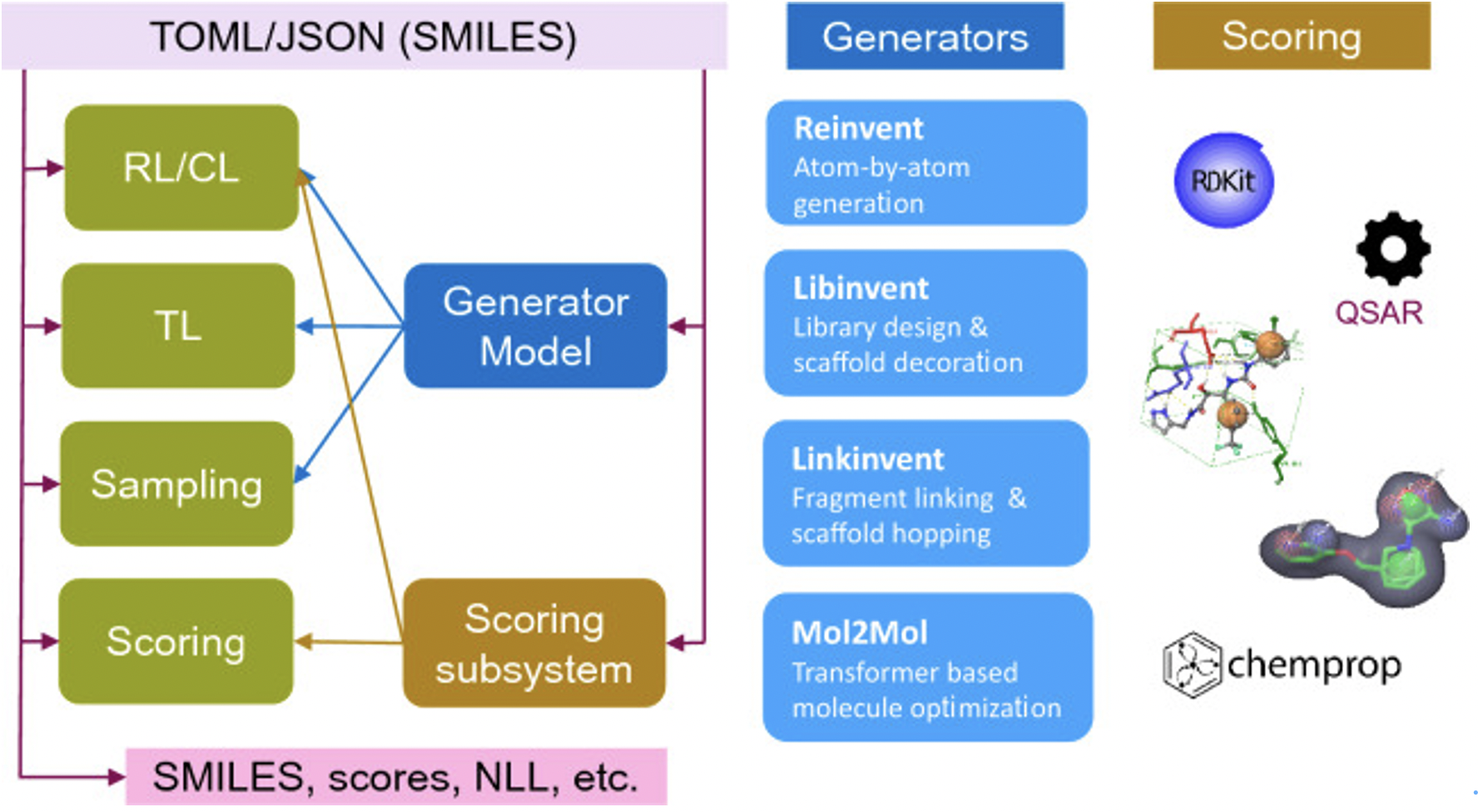
Some AI software platforms are specifically designed for the design of small nucleic acid drugs. For instance, certain AI tools are utilized within modern open-source generative AI frameworks for small molecule design. These tools employ recurrent neural networks and transformer architectures to drive molecular generation, supporting and facilitating de novo design, R-group modification, library design, linker design, skeleton hopping, and molecular optimization.
In the realm of small nucleic acid drug discovery, AI plays a multifaceted role. It analyzes vast amounts of chemical and biomedical data to predict the activity and pharmacological properties of small nucleic acid drugs, thereby accelerating the identification of potential drug candidates. AI can predict the three-dimensional structure of these drugs, which is crucial for understanding their interactions with targets. Moreover, AI rapidly screens numerous compounds to identify potential drug candidates, optimizes the biological effects of drug molecules, and even repurposes drugs by predicting unexpected activities. These advancements provide new strategies and tools for drug development, particularly in the field of small nucleic acid drugs, where AI applications help enhance the targeting and efficacy of drugs.
AI-driven platforms for RNA therapy offer a range of services, including the design of siRNA drugs, small molecule drugs, and RNA vaccines. These platforms include modules such as RNA-finder, RNA-structure, RNA-target, and RNA-med, which are designed to advance the development of RNA therapies through AI technology. RNA-finder is utilized for the discovery of novel noncoding RNAs, while RNA-structure predicts RNA secondary structures. The RNA-target module reveals the targets of RNA, and RNA-med focuses on AI-based RNA modeling for medical applications. These platforms aim to streamline the process of drug design and target identification, making the development of small nucleic acid drugs more precise and efficient.
Patsnap Bio's Role in the Development of Small Nucleic Acid Drugs
Patsnap Bio plays a crucial role in the development of small nucleic acid drugs. By comprehensively collecting and deeply processing protein and nucleic acid sequence data from global patents, biological journals, and public biological databases, it provides researchers with a powerful tool to accelerate the discovery and validation of drug targets.
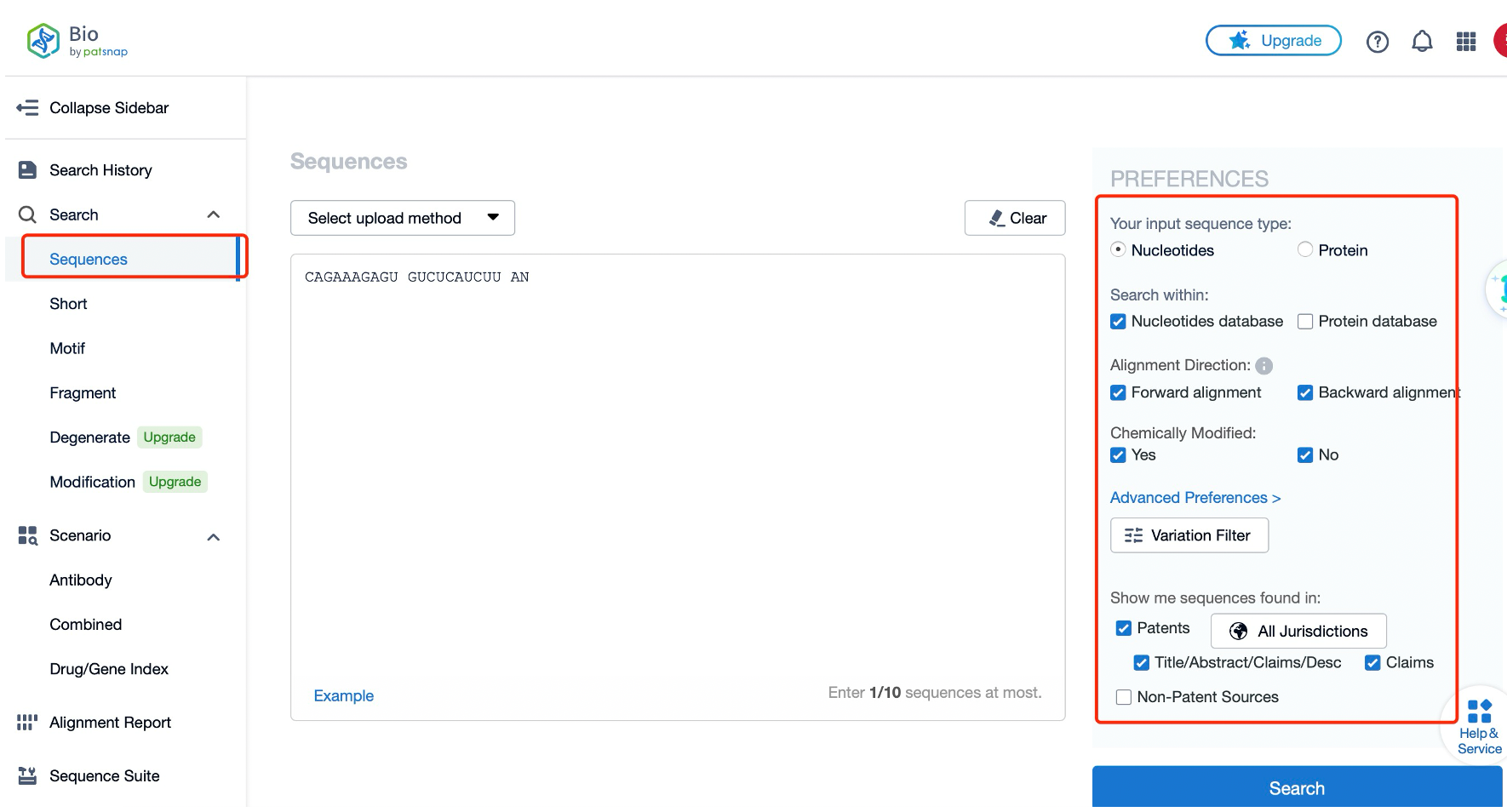
Firstly, the manual annotations and modified structure annotations in Patsnap Bio allow researchers to quickly obtain accurate information on key biological sequences, enhancing the efficiency of sequence information acquisition. Moreover, the database associate’s multi-dimensional information such as biological sequences, species, drugs, diseases, structures, and functions through knowledge graphs, which is essential for understanding the mechanisms of action of small nucleic acid drugs and optimizing drug design.
Secondly, the various advanced sequence alignment algorithms provided by Patsnap Bio can assist researchers in quickly searching and analyzing known similar sequences globally, thereby grasping risk and competitive intelligence. This is extremely convenient for the early stages of small nucleic acid drug development, especially in sequence modification analysis, existing technology research, innovation analysis, and risk avoidance.
Patsnap Bio's Role in the Chemical Modification of Small Nucleic Acid Drugs
The application of Patsnap Bio in the chemical modification of small nucleic acid drugs has significantly enhanced the efficiency and precision of research and development in this field. By introducing the world's first chemical modification search function, Patsnap Bio allows researchers to directly search for sequences and patent information related to specific chemical modifications. For instance, 2'-O-methyl (2'-OMe) modification is a common method for modifying small nucleic acid drugs. With this feature, users can quickly locate all sequences containing such modifications without having to sift through individual descriptions in the literature. This innovation not only saves a considerable amount of time and effort but also improves the accuracy and comprehensiveness of the search, as the function covers a wide range of modification types and has been standardized.
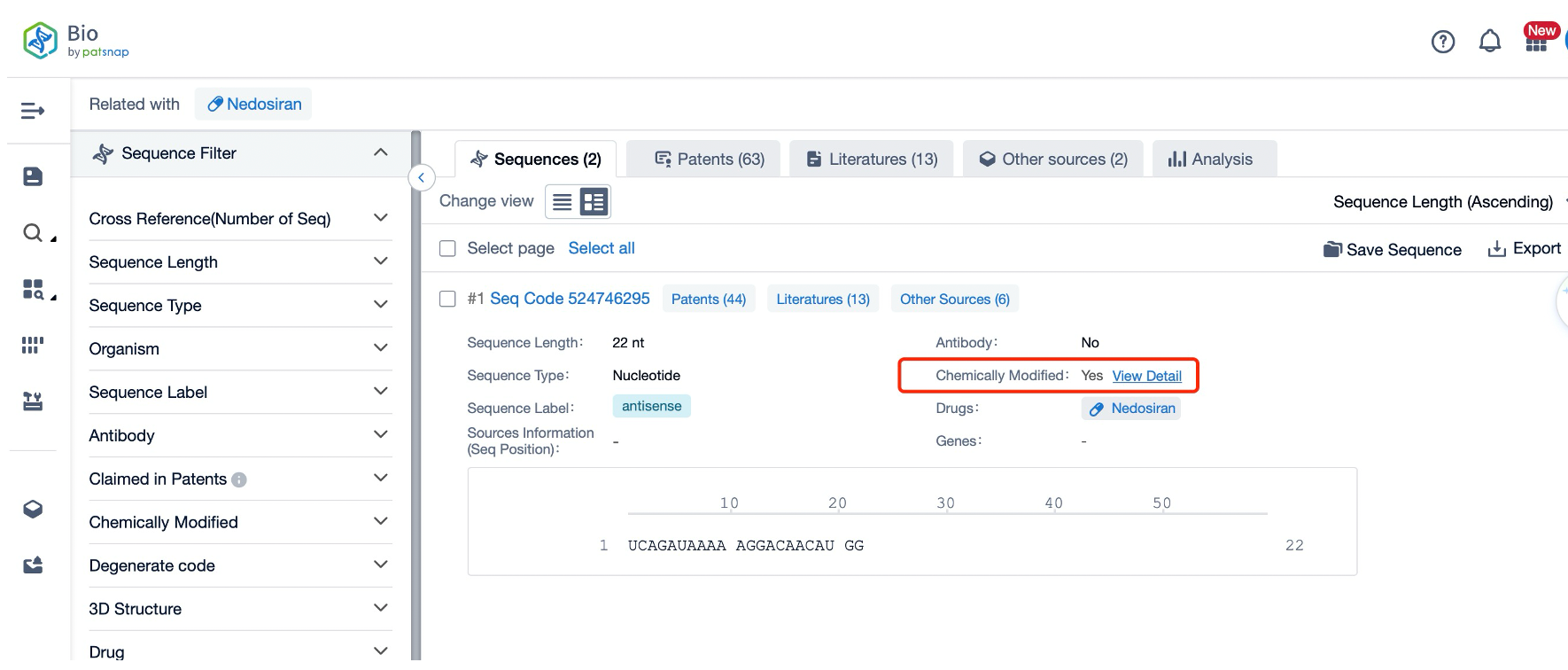
Sequence Database plays a crucial role in addressing the inconsistency in the description of chemical modifications in patent literature. Traditionally, the lack of a unified standard has made it necessary for researchers to manually include all possible modification terms when searching with keywords, which is not only complex but also prone to omitting important information. By establishing a standardized search system that covers nine major categories and 382 subcategories of chemical modifications, Patsnap Bio has achieved consistent processing of modification information. For instance, users can directly select the desired modification types on the search interface without worrying about incomplete results due to term variances. This innovation not only saves a significant amount of time and effort but also enhances the accuracy and comprehensiveness of the search, as the function includes a broad range of modification types and has undergone standardization processing.
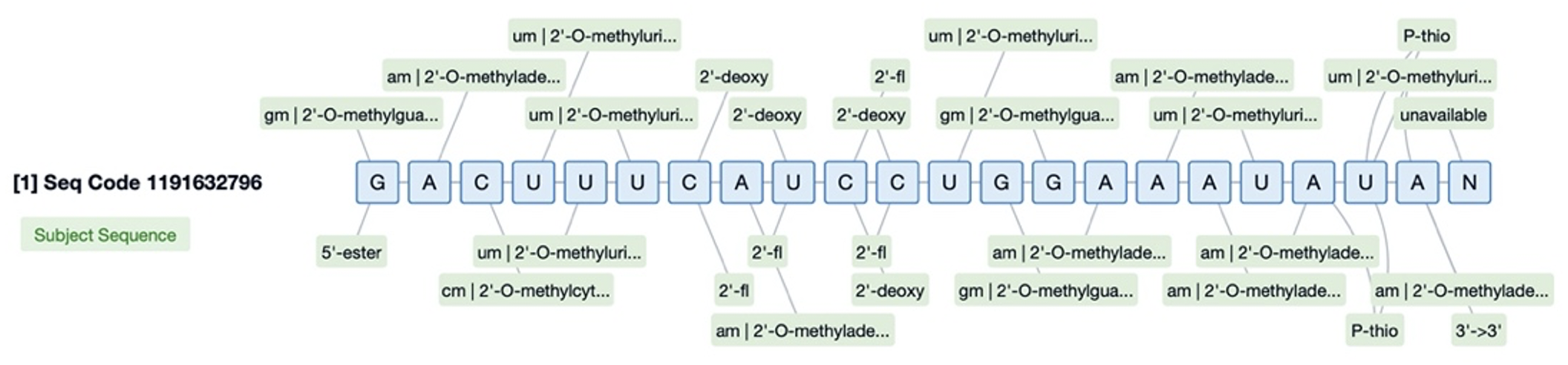
In addition to its role in chemical modification, Patsnap Bio plays a significant role in the chemical modification of small nucleic acid drugs, greatly enhancing the efficiency and precision of research and development in this field. By introducing the world's first chemical modification search function, Patsnap Bio allows researchers to directly search for sequences and patent information related to specific chemical modifications. For instance, 2'-OMe modification is a common method for modifying small nucleic acid drugs. With this feature, users can quickly find all sequences containing such modifications without having to browse through descriptions in the literature one by one. This innovation not only saves a lot of time and effort but also improves the accuracy and comprehensiveness of the search because the function covers a wide range of modification types and has been standardized.
Furthermore, Patsnap Bio's multi-dimensional analysis tools associate chemical modification information with various levels of data such as biological sequences, species, drugs, diseases, structures, and functions. This comprehensive presentation of information allows researchers to examine the mechanisms of action of chemical modifications and their applications in drug design from different perspectives. For example, when searching for candidate drugs to treat specific diseases, scientists can use the database to analyze which chemical modifications may enhance the stability and targeting of drugs, thereby optimizing drug design schemes.
In terms of intellectual property protection, the freedom-to-operate (FTO) assessment function provided by Patsnap Bio for chemically modified sequences brings great convenience to IP professionals. For instance, when assessing the FTO risks of a new type of small nucleic acid drug, Patsnap Bio can help users quickly identify potential patent infringement risks and ensure that research and development activities do not infringe on others' patent rights through detailed comparative analysis. This not only safeguards the legality of research results but also provides solid legal support for the subsequent commercial operations of the company.
"Hiro-LS” aids the development of small nucleic acid drugs
The large-scale biopharmaceutical model "Hiro-LS" enhances the efficiency and precision of research and development in the field of small nucleic acids. By analyzing vast biomedical data, Hiro-LS can predict the activity and pharmacological properties of small nucleic acid drugs, thus accelerating the discovery of potential drug candidates.
Hiro-LS 's manual annotations and modified structure annotations allow researchers to quickly obtain accurate biological sequence information, improving the efficiency of sequence retrieval. It uses knowledge graphs to associate biological sequences with various dimensions of data, including species, drugs, diseases, structures, and functions. This is crucial for understanding the mechanisms of action of small nucleic acid drugs and optimizing drug design.
Hiro-LS also offers advanced sequence alignment algorithms that help researchers quickly search and analyze known similar sequences globally. This capability is beneficial for early-stage drug development, including sequence modification analysis, existing technology research, innovation analysis, and risk avoidance.
In terms of intellectual property protection, Patsnap Bio provides a FTO assessment feature for chemically modified sequences. This feature is particularly useful for IP professionals as it helps quickly identify potential patent infringement risks and ensures that research activities do not infringe upon others' patent rights.
Moreover, Patsnap Bio's multi-dimensional analysis tools link chemical modification information with various levels of data, enabling researchers to examine the mechanisms of action of chemical modifications and their applications in drug design from different perspectives. For instance, when searching for candidate drugs to treat specific diseases, scientists can use the database to analyze which chemical modifications might enhance the stability and targeting of drugs, thus optimizing drug design schemes.
In the realm of clinical development, Hiro-LS can comprehensively monitor and summarize the progress of small nucleic acid drugs. By systematically organizing information on clinical stages and indication expansion, Patsnap provides a clear picture of the R&D landscape. Particularly, the tracking and analysis of leading companies' product lines, such as Ionis and Alnylam, along with in-depth studies of successful cases, offer significant references for other companies' R&D decisions. Overall, Patsnap, through its multi-dimensional and in-depth integration and analysis of information, plays an indispensable role as a think-tank in advancing the development of small nucleic acid drugs.
For an experience with the large-scale biopharmaceutical model Hiro-LS, please click here for a quick and free trial of its features!
summary
AI is gradually transforming the traditional paradigms in the field of small nucleic acid drug development. From drug target discovery to compound screening and drug design, AI, through ML and DL technologies, has significantly improved the efficiency and precision of drug development. For instance, AI technology can predict the three-dimensional structure of small nucleic acid drugs, aiding in understanding the interactions between drugs and targets; at the same time, AI can also screen potential drug candidates from massive datasets, optimizing the biological effects of drug molecules. Additionally, AI plays a crucial role in optimizing drug delivery systems by predicting the best delivery pathways and carriers, enhancing the stability and delivery efficiency of small nucleic acid drugs. Several international databases and software platforms provide a rich data foundation for training AI models, and the combination of these resources with AI technology not only accelerates the drug discovery and screening process but also provides strong support for future drug development.
Patsnap Bio plays a significant role in the development of small nucleic acid drugs. With its unique chemical modification search function and standardized modification system, it greatly enhances the efficiency and accuracy of chemical modification sequence information retrieval. The database not only provides authentic information on key biological sequences but also associates biological sequences, species, drugs, diseases, and other multi-dimensional information through knowledge graphs, providing multi-angle analytical tools for understanding the mechanisms of action of small nucleic acid drugs. Furthermore, the multi-dimensional analysis tools and advanced sequence alignment algorithms of Patsnap Bio help researchers quickly search and analyze known global similar sequences, thereby better grasping risks and competitive intelligence. The application of these tools in sequence modification analysis and existing technology research has significantly improved the efficiency of small nucleic acid drug development.
The large-scale biopharmaceutical model "Hiro-LS," provides comprehensive support for the development of small nucleic acid drugs at multiple levels, including market analysis, technology research, and clinical progress. By continuously tracking and analyzing the global market landscape, key enterprise product pipelines, "Hiro-LS" offers valuable market insights to the industry. On the technical level, "Hiro-LS" focuses on key challenges such as delivery technology and drug stability, and by analyzing innovative solutions, it provides valuable references for solving technical difficulties. In terms of clinical development, "Hiro-LS" systematically organizes information on clinical stages and indication expansion, providing important basis for other enterprises' R&D decisions. In summary, the multi-dimensional information integration and analysis capabilities of Patsnap's AI tool "Hiro-LS" inject new momentum into the development process of small nucleic acid drugs.
References:
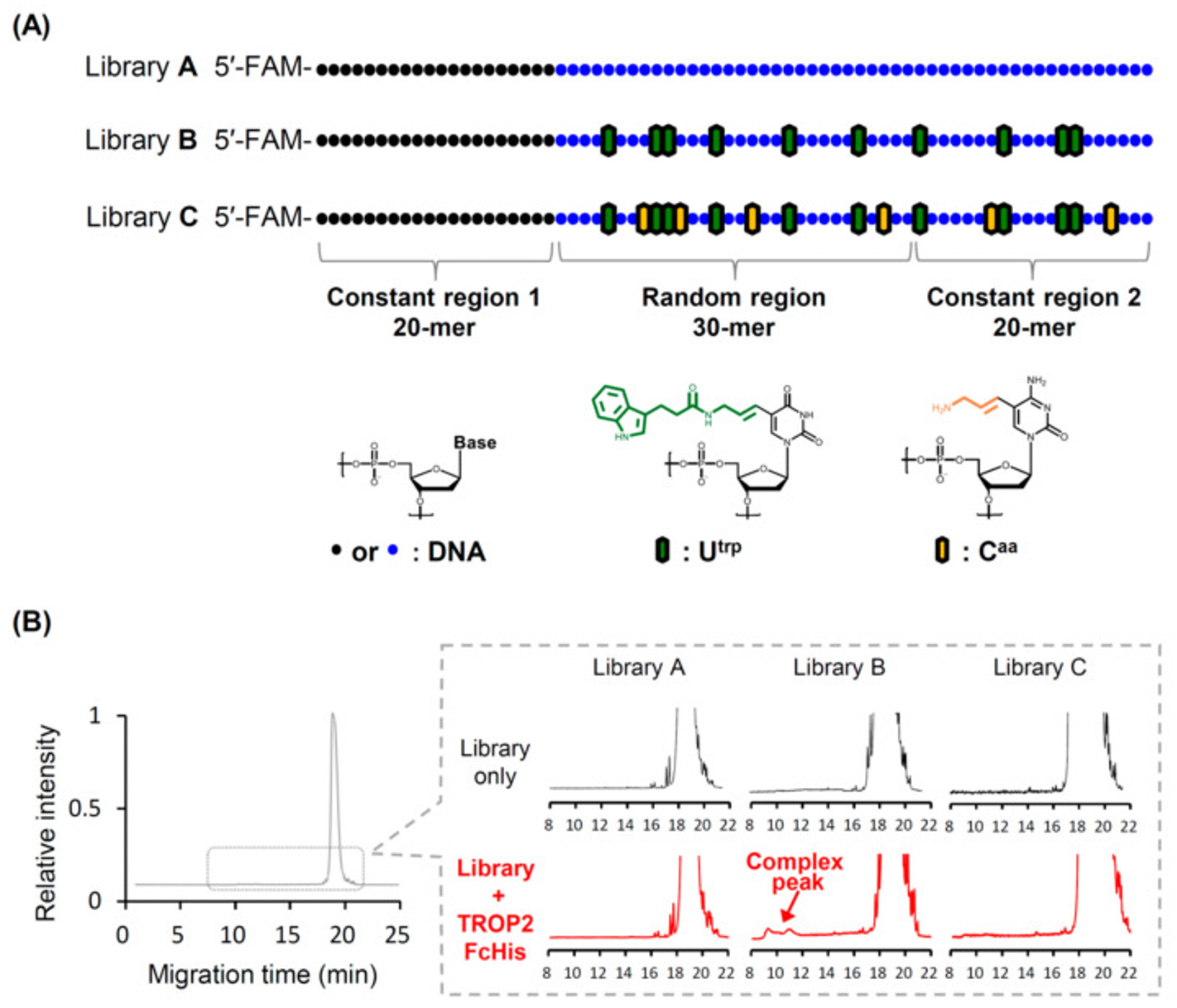
- 1.Uemachi, H., et al., Hybrid-Type SELEX for the Selection of Artificial Nucleic Acid Aptamers Exhibiting Cell Internalization Activity. Pharmaceutics, 2021. 13(6).
- 2.Knox, C., et al., DrugBank 6.0: the DrugBank Knowledgebase for 2024. Nucleic Acids Res, 2024. 52(D1): p. D1265-D1275.
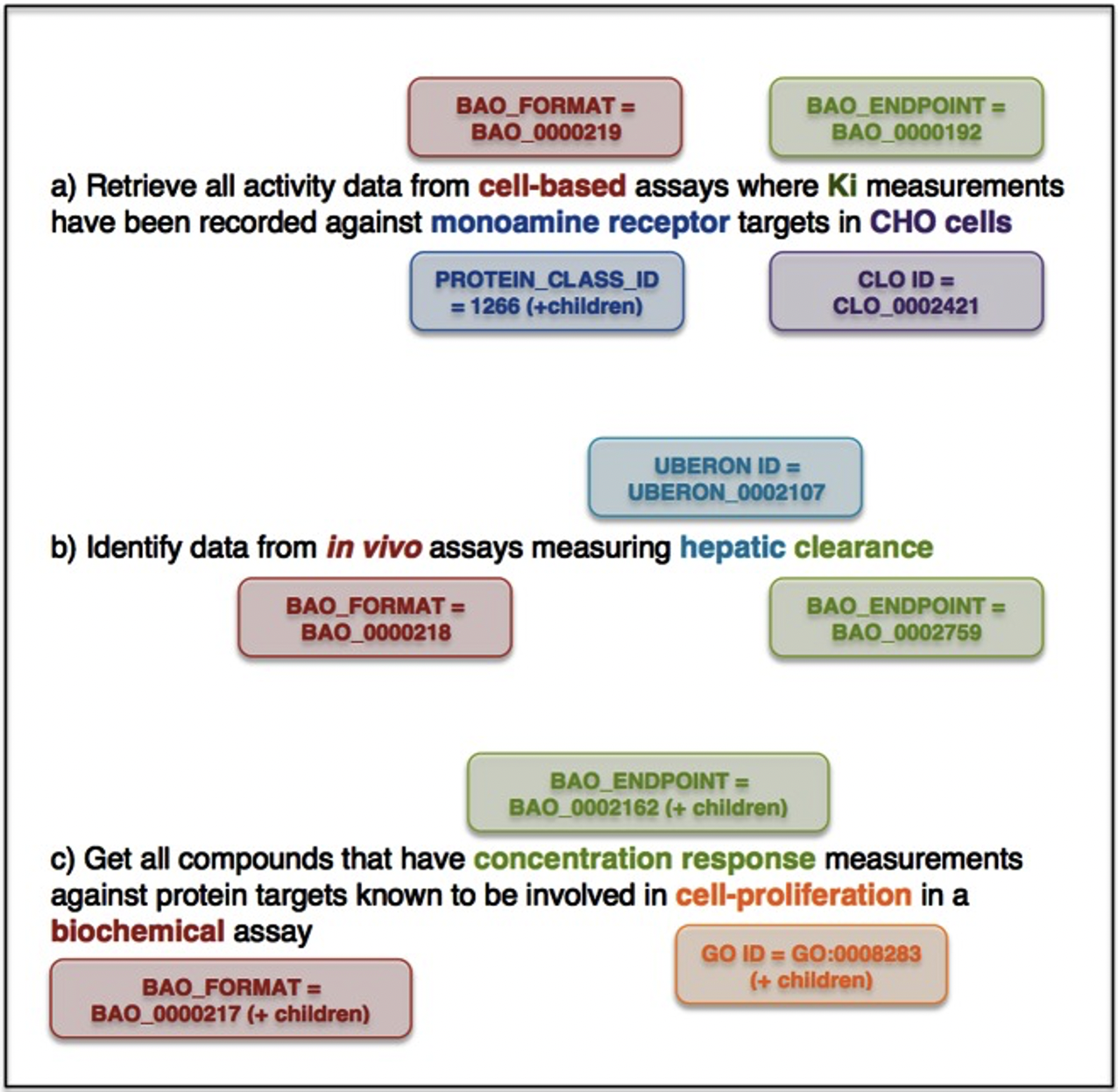
- 3.Mendez, D., et al., ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res, 2019. 47(D1): p. D930-D940.
- 4.Loeffler, H.H., et al., Reinvent 4: Modern AI-driven generative molecule design. J Cheminform, 2024. 16(1): p. 20.