How to find the core components of mylotarg?
Mylotarg also known as gemtuzumab ozogamicin, is a pioneering antibody-drug conjugate (ADC) developed by Pfizer, targeting the CD33 antigen, which is frequently expressed on the surface of acute myeloid leukemia (AML) cells. Initially approved by the FDA in 2000, mylotarg was the first ADC to enter the market, marking a significant milestone in the field of targeted cancer therapy. It was developed to deliver a potent antitumor agent, calicheamicin, directly to CD33-expressing leukemia cells.
Summary of Research Progress of Mylotarg
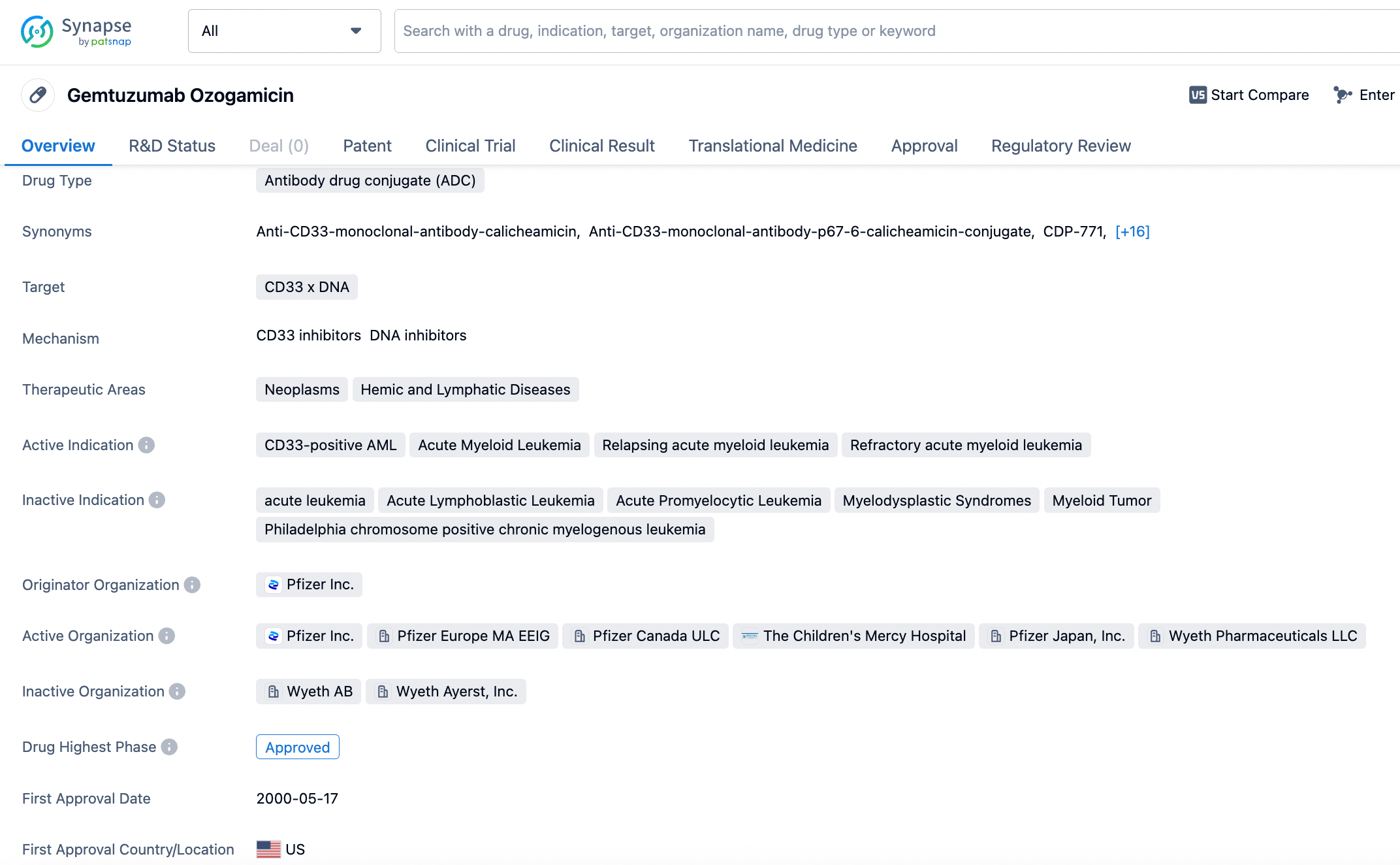
Mylotarg's initial approval was based on its potential to deliver the potent anti-tumor agent calicheamicin directly to CD33-expressing AML cells. However, due to safety concerns and the inability to confirm clinical benefits in subsequent trials, it was voluntarily withdrawn from the market by Pfizer in 2010. Despite this setback, the drug's potential was not overlooked. After extensive research and dose titration, mylotarg was reapproved by the FDA in 2017 for the treatment of newly diagnosed and relapsed or refractory CD33-positive AML in adults, as well as in pediatric patients aged two and above.
The global competitive landscape for CD33-targeted therapies is relatively focused, with mylotarg being the only approved ADC against this antigen. However, the broader market for AML treatments includes various chemotherapy regimens and other targeted therapies. The reapproval of mylotarg has reignited interest in ADCs for AML, and while it currently stands alone in its niche, the field is active with research into new compounds and approaches.
1.Antibody Binding: The humanized monoclonal antibody component of mylotarg has a high affinity for the CD33 antigen, which is expressed on the surface of most AML cells. This specificity ensures that the drug is primarily delivered to leukemic cells while sparing healthy cells that do not express CD33.
2.Internalization: Once bound to the CD33 antigen, the mylotarg-CD33 complex is internalized by the leukemic cells through endocytosis. This internalization is a critical step in delivering the cytotoxic payload directly to the targeted cells.
3.Payload Release: Inside the cell, the calicheamicin payload is released through proteolytic cleavage of the linker connecting the antibody and the drug. Calicheamicin is a highly potent toxin that was originally isolated from bacteria and is known for its ability to induce double-stranded breaks in DNA.
4.DNA Damage and Cell Death: Upon release, calicheamicin localizes to the nucleus and induces severe DNA damage. This damage triggers a series of events that ultimately lead to the apoptosis (programmed cell death) of the leukemic cells.
By leveraging the precision of the monoclonal antibody to deliver a powerful cytotoxic agent directly to the site of action, mylotarg aims to minimize systemic side effects while maximizing the therapeutic effect on the targeted cancer cells. This targeted delivery mechanism makes mylotarg a valuable tool in the treatment of AML, particularly in scenarios where traditional chemotherapy might be less effective or associated with higher toxicity.
Structural Characteristics of Mylotarg
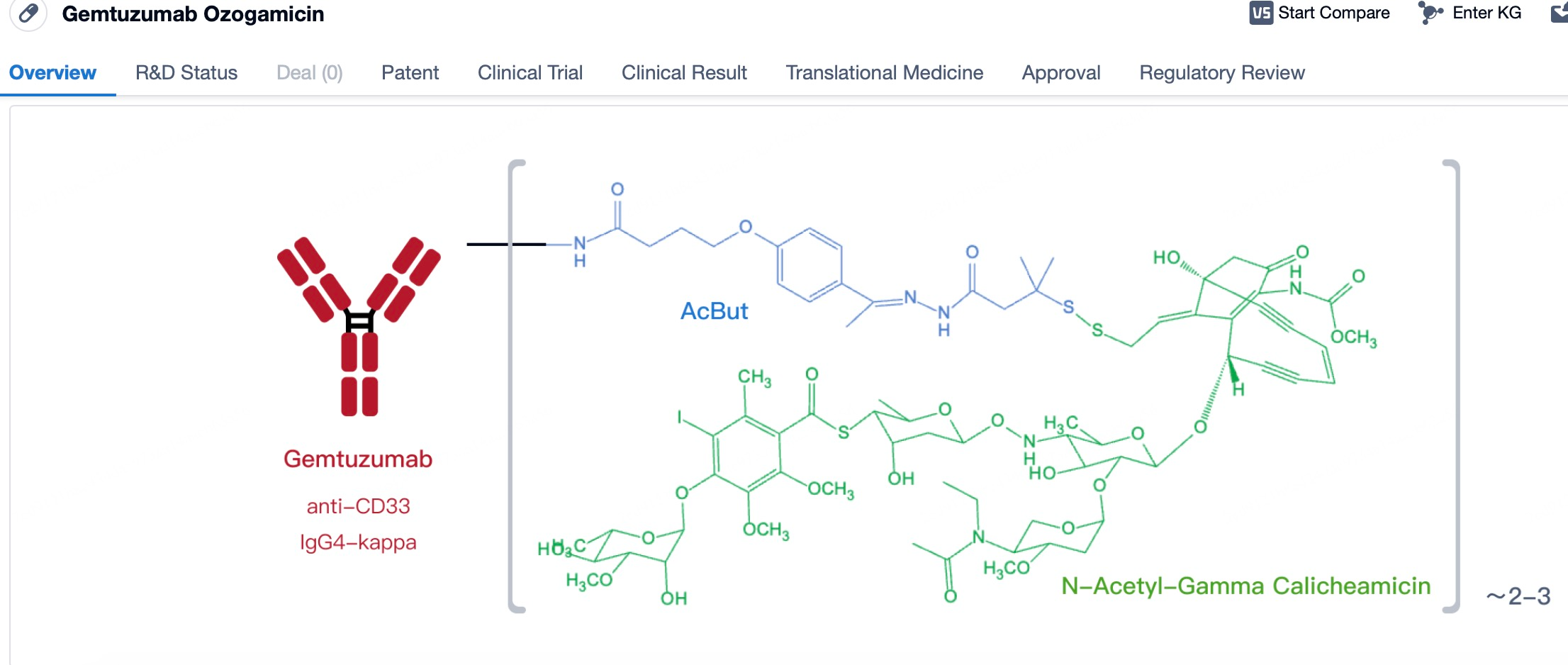
Structurally, mylotarg is characterized by its humanized IgG4 backbone, which contributes to its stability and reduced immunogenicity. The calicheamicin is attached to the antibody via a linker system that is designed to be stable in the bloodstream but can be cleaved within the target cells to release the active drug.
These structural features collectively contribute to the therapeutic efficacy and safety profile of mylotarg, enabling it to deliver a potent cytotoxic payload directly to the cancer cells while limiting systemic toxicity.
1)Selection and Advantages of Mylotarg Antibodies
The selection of the anti-CD33 antibody for use in mylotarg was a strategic decision based on the high expression of the CD33 antigen on the surface of myeloid cells, particularly those involved in acute myeloid leukemia (AML). CD33 is a transmembrane receptor expressed on hematopoietic progenitors and differentiated myeloid cells, including monocytes, granulocytes, and a subset of lymphoid cells. However, its expression is notably elevated in the majority of AML cases, making it an attractive target for therapeutic intervention.
2)Advantages of Targeting CD33:
1.Specificity: The high specificity of the anti-CD33 antibody for AML cells reduces the likelihood of off-target effects, thus minimizing damage to healthy cells and tissues. This specificity translates into a better safety profile compared to traditional chemotherapies, which often affect rapidly dividing normal cells as well.
2.Reduced Toxicity: By targeting CD33-positive cells, mylotarg can deliver a potent cytotoxic payload directly to the cancer cells while sparing healthy tissues that lack the CD33 antigen. This targeted approach helps to reduce the systemic side effects typically associated with conventional chemotherapy.
3.Enhanced Efficacy: The conjugation of calicheamicin to the anti-CD33 antibody creates a highly effective therapeutic agent that can induce apoptosis in AML cells through the induction of DNA damage. The potency of calicheamicin combined with the targeting capability of the antibody allows for a concentrated and potent anti-leukemia effect.
4.Patient Selection: Given the expression pattern of CD33, mylotarg can be used in a well-defined subset of AML patients, providing a personalized medicine approach that can tailor treatment to those most likely to benefit from the therapy.
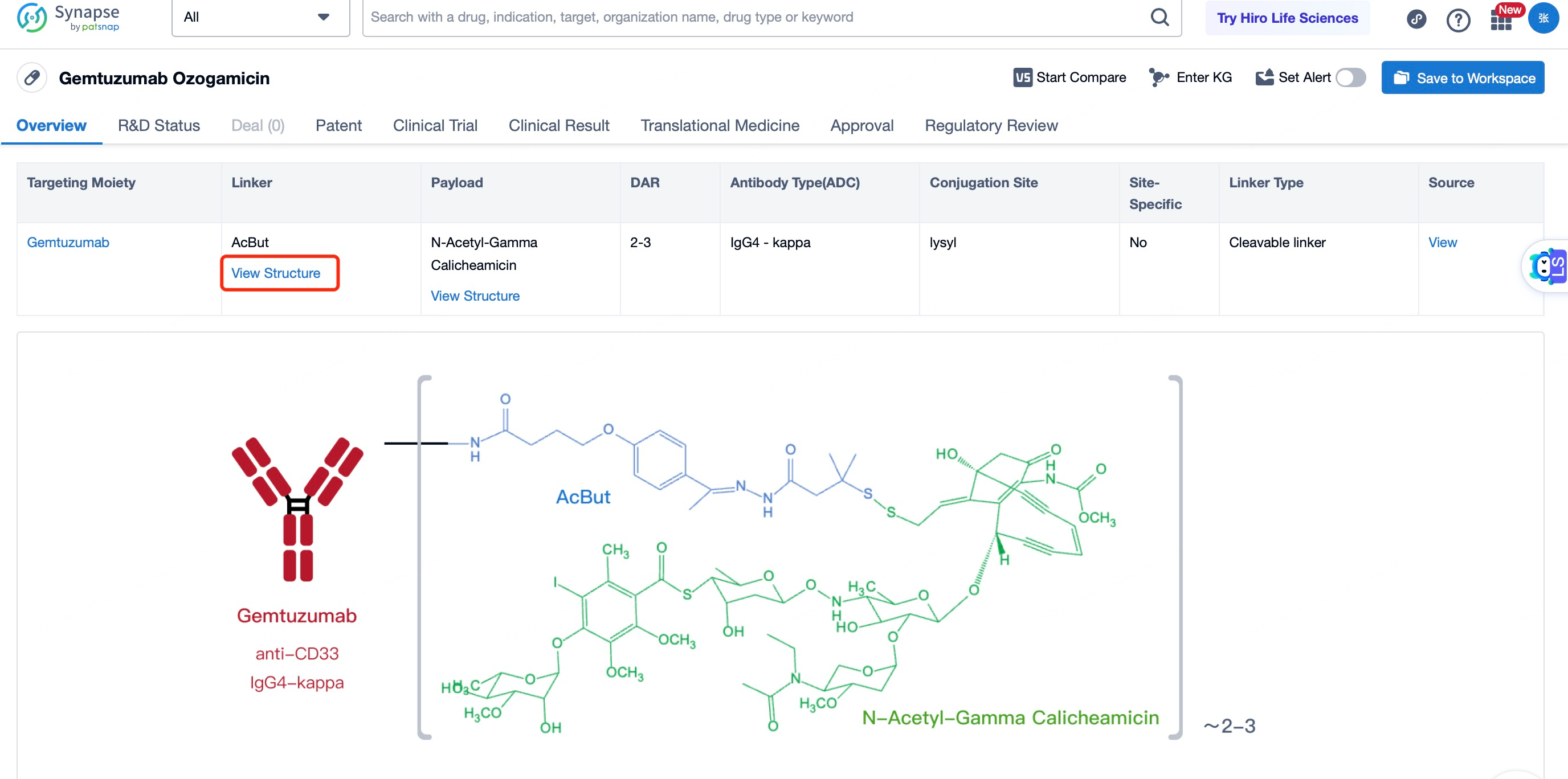
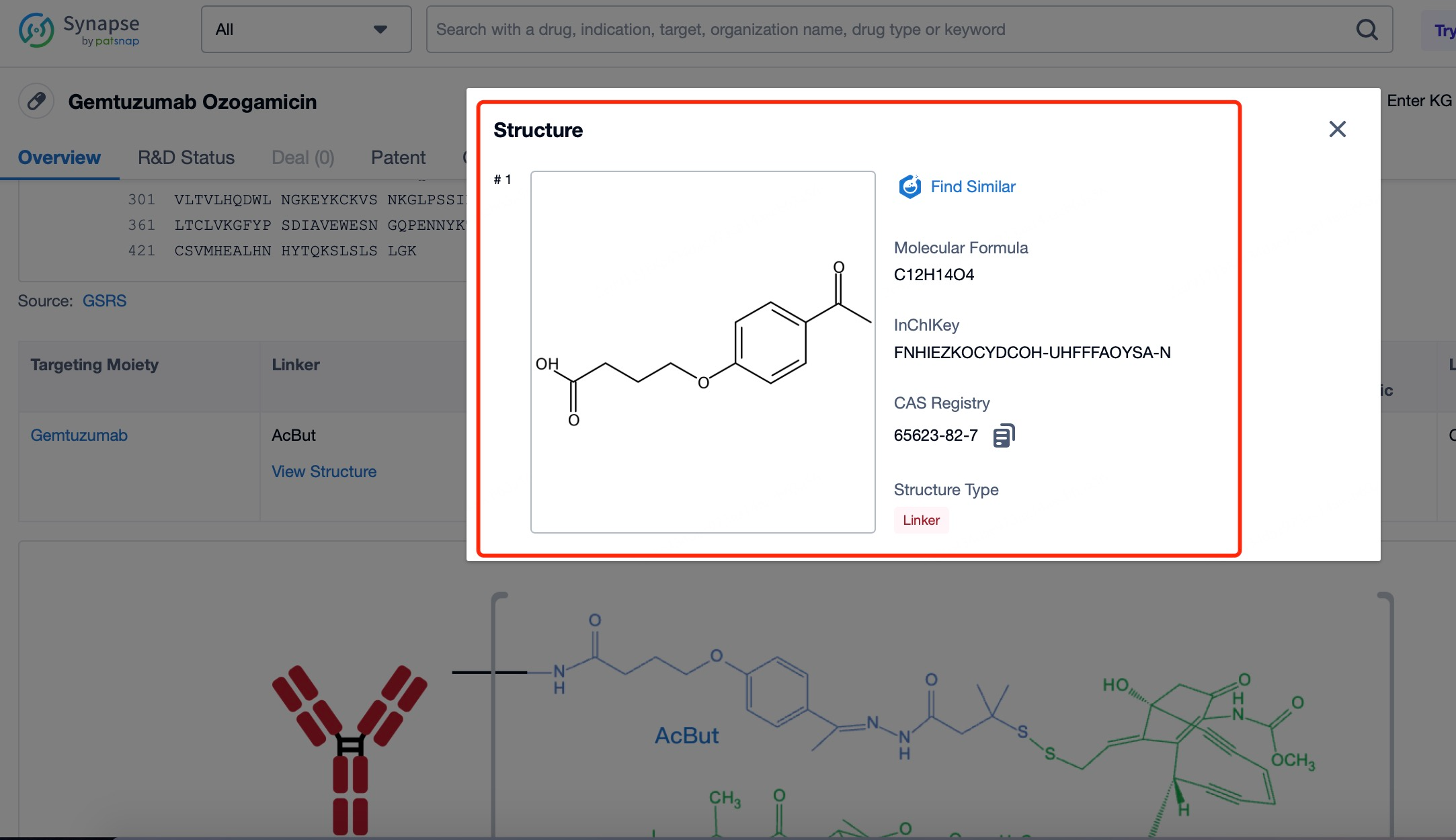
3)Linker of Mylotarg
The linker in mylotarg plays a pivotal role in the therapeutic efficacy and safety profile of the drug. Initially, concerns over the stability of the linker led to the withdrawal of mylotarg; however, subsequent redesign efforts have resulted in a linker that is both stable in circulation and optimized for efficient drug release within the target cells. The current linker system is engineered to remain intact in the bloodstream, ensuring that the cytotoxic payload remains attached to the antibody until it reaches the intended target. Once the antibody-drug conjugate (ADC) is internalized by CD33-expressing cells, the linker is designed to be cleaved under specific intracellular conditions, such as changes in pH or the presence of specific enzymes, allowing the release of the highly potent calicheamicin directly into the cancer cell cytoplasm. This precise timing and localization of drug release minimize the potential for off-target toxicity, thereby enhancing the therapeutic index of mylotarg. The optimized linker thus represents a critical advancement in balancing the stability required during systemic circulation with the controlled release necessary for effective cancer cell killing.
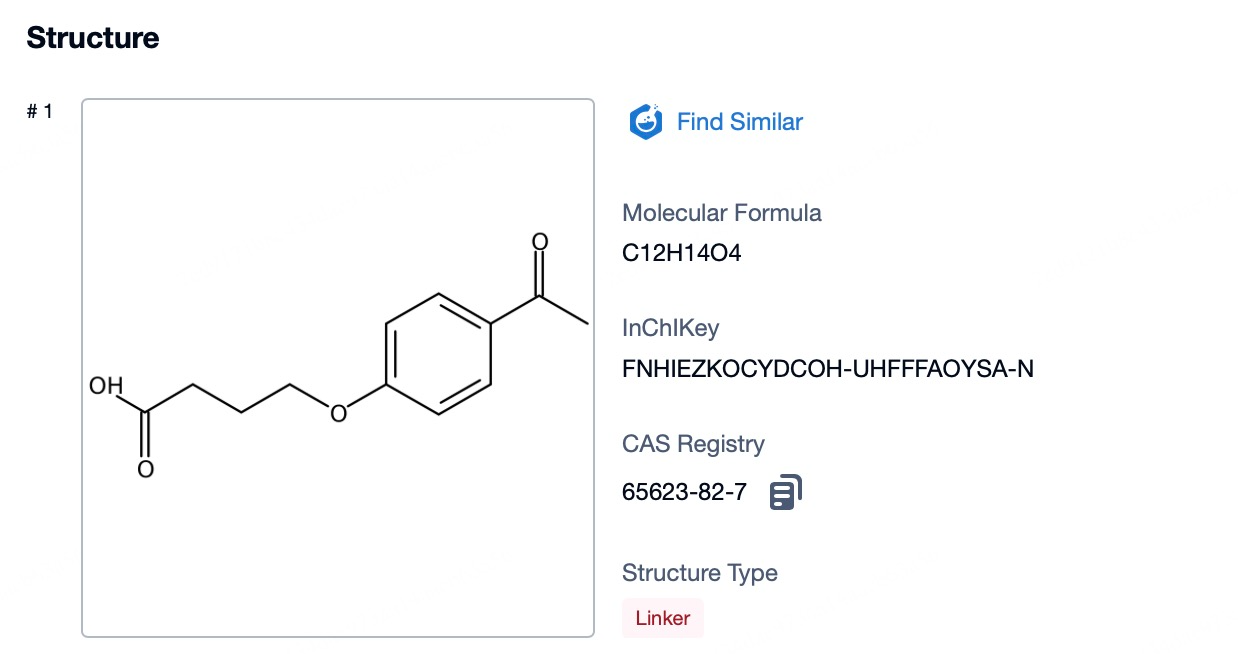
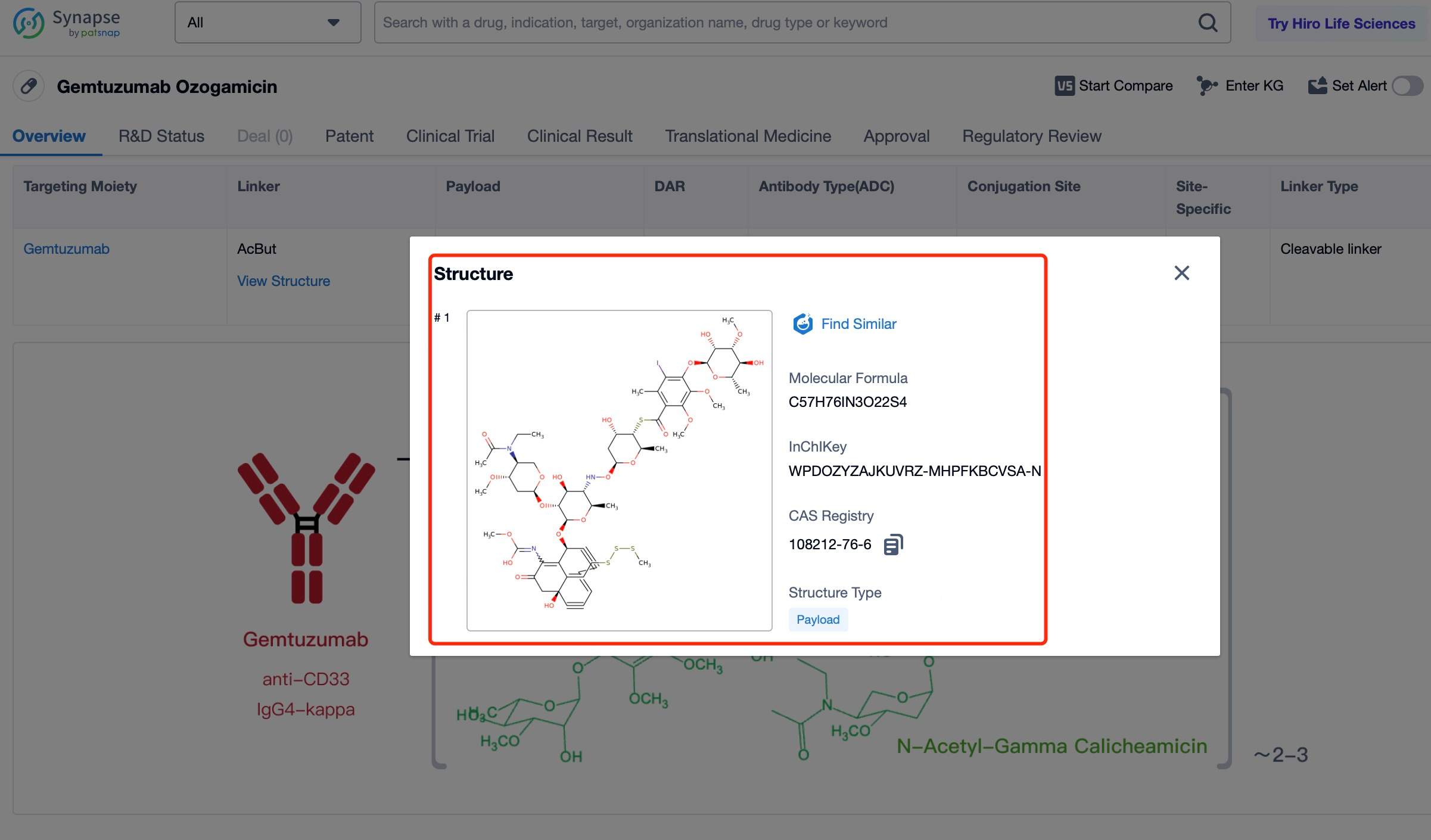
4)Characteristics of Cytotoxic Drug Payloads in Mylotarg
The cytotoxic drug payload of mylotarg, calicheamicin, is a highly potent DNA-binding agent known for its remarkable efficacy in inducing cell death. Calicheamicin's potency lies in its ability to create double-stranded breaks in DNA, a lethal form of damage that leads to the rapid demise of the targeted cancer cells. Because of its high potency, only minute quantities of calicheamicin are necessary to achieve a significant antitumor effect, which is a key advantage of antibody-drug conjugates (ADCs) like mylotarg. This characteristic allows for the targeted delivery of a powerful drug directly to the cancer cells, minimizing the dose required and thereby reducing the potential for systemic side effects. The precision of calicheamicin's action, coupled with the specificity of the anti-CD33 antibody, ensures that the drug payload is efficiently delivered to the site of action, maximizing its therapeutic impact while sparing surrounding healthy tissue. This targeted approach is designed to improve the overall safety and effectiveness of cancer therapy, offering patients a more tailored and less toxic treatment option.
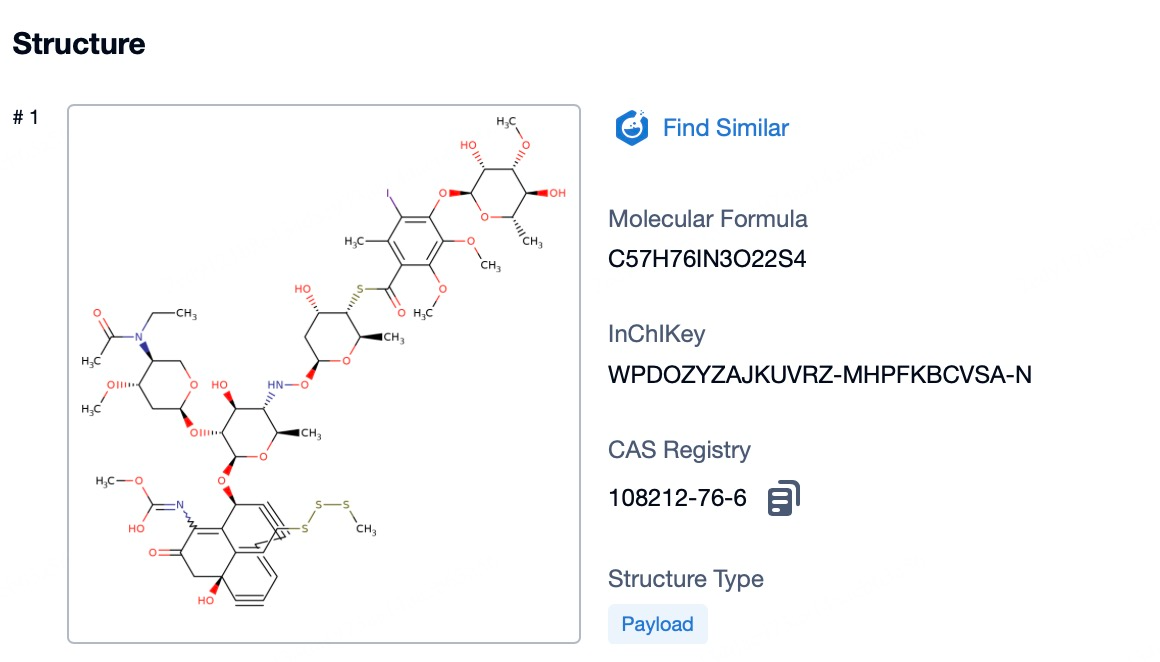
Summary and Prospect
In summary, mylotarg's journey from the first ADC approval to withdrawal and reapproval highlights the dynamic nature of drug development. Its current market position is a testament to the importance of targeted therapies in oncology. Looking forward, mylotarg is expected to continue to play a role in the treatment of AML, particularly in patient populations where its targeted approach is most beneficial. The future of mylotarg may also include further research into additional indications or combination therapies, based on the ongoing understanding of CD33 expression in other cancers.
How to find the the core components of an ADC drug?
In PatSnap Synapse and PatSnap Bio, you can find the sequence and latest research and development advances of all ADC drugs.
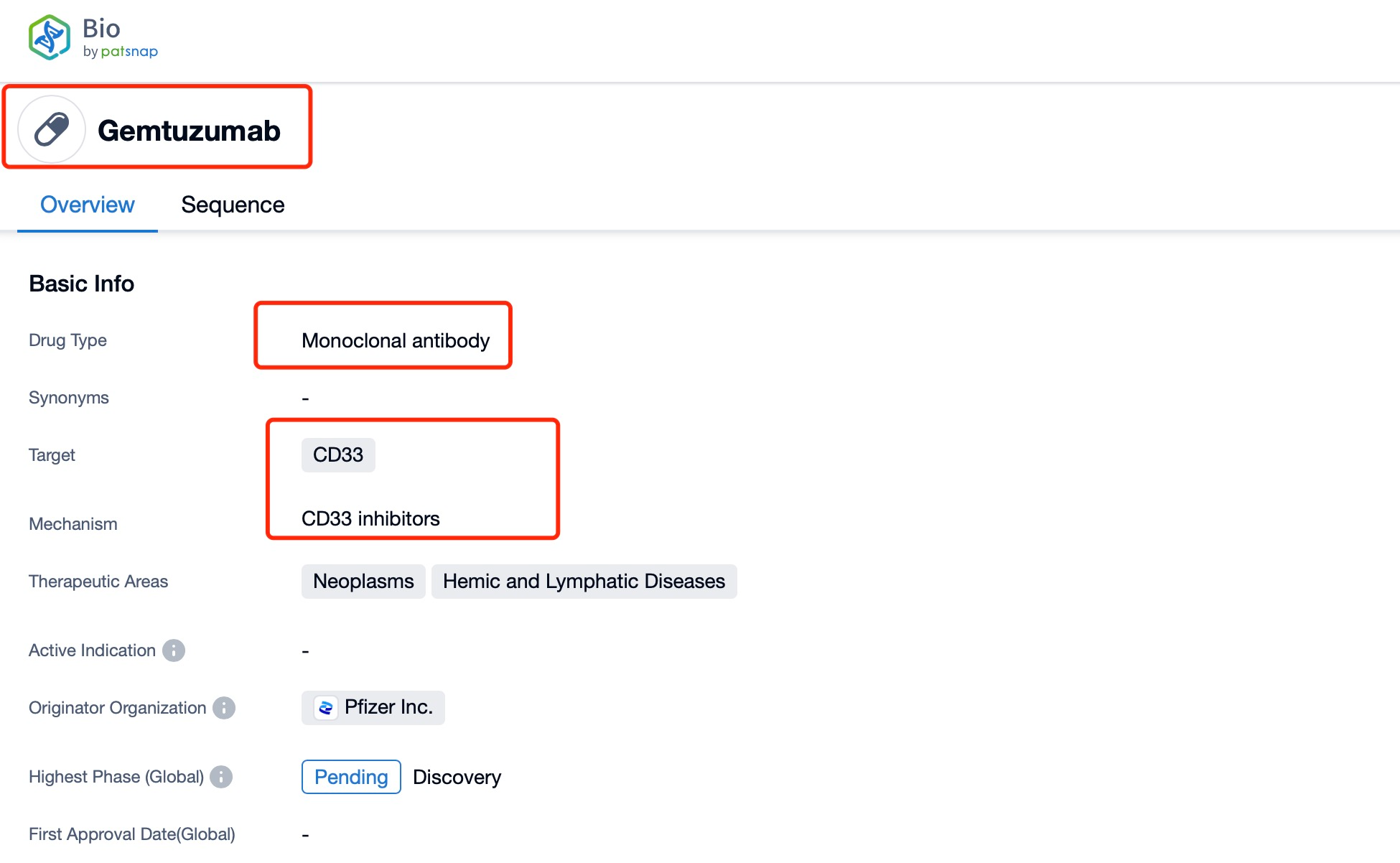
Taking Mylotarg as an example, First, you can log in to PatSnap Synapse, enter 'Mylotarg' in the search box and click to view the details. On the details page, you can find the basic information and research progress of Mylotarg.
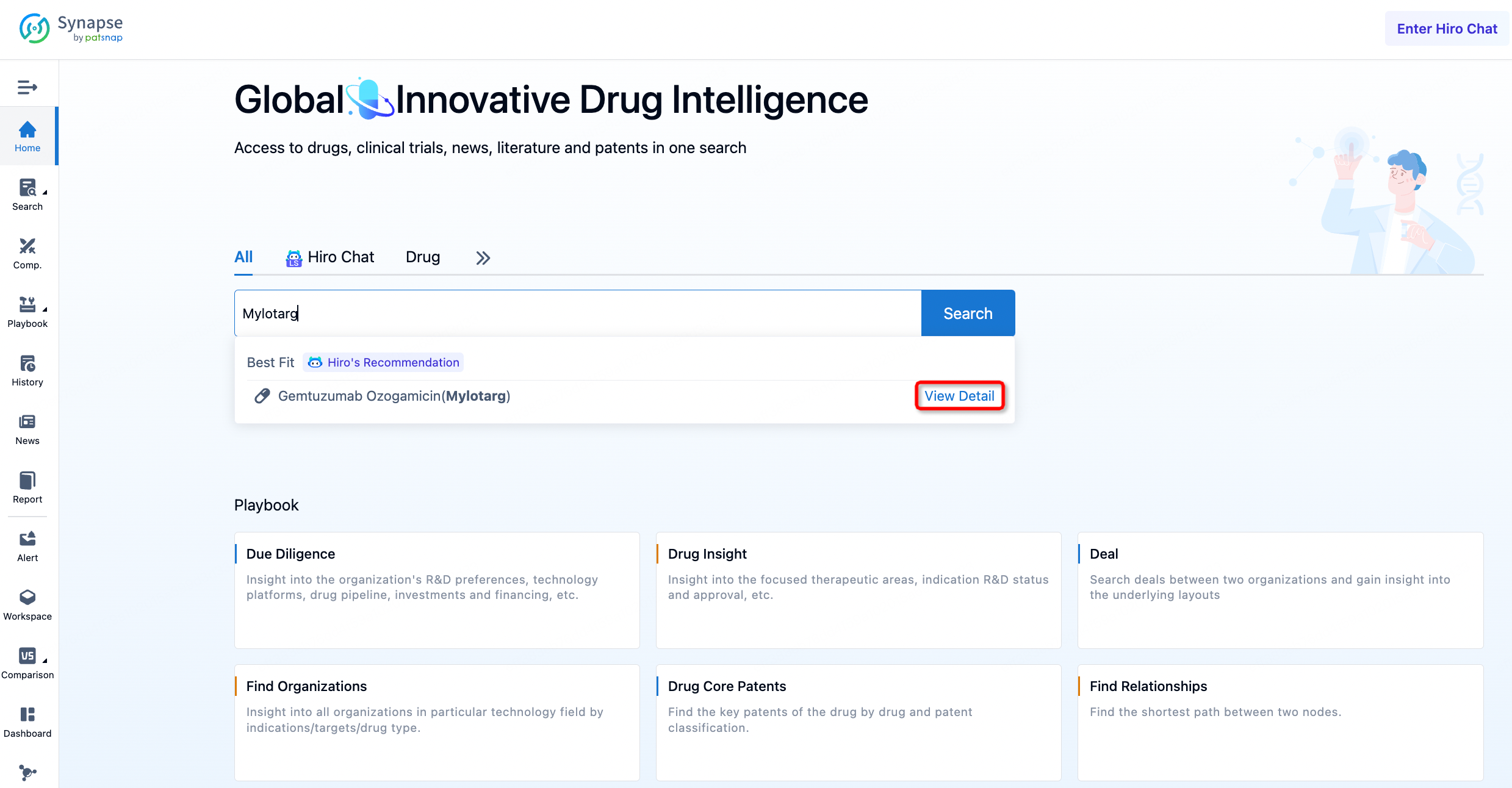
After entering the details page, drop down to find the core Structure information of ADC drug and click view Structure in the Linker section to find the structure and type of ADC drug Linker.
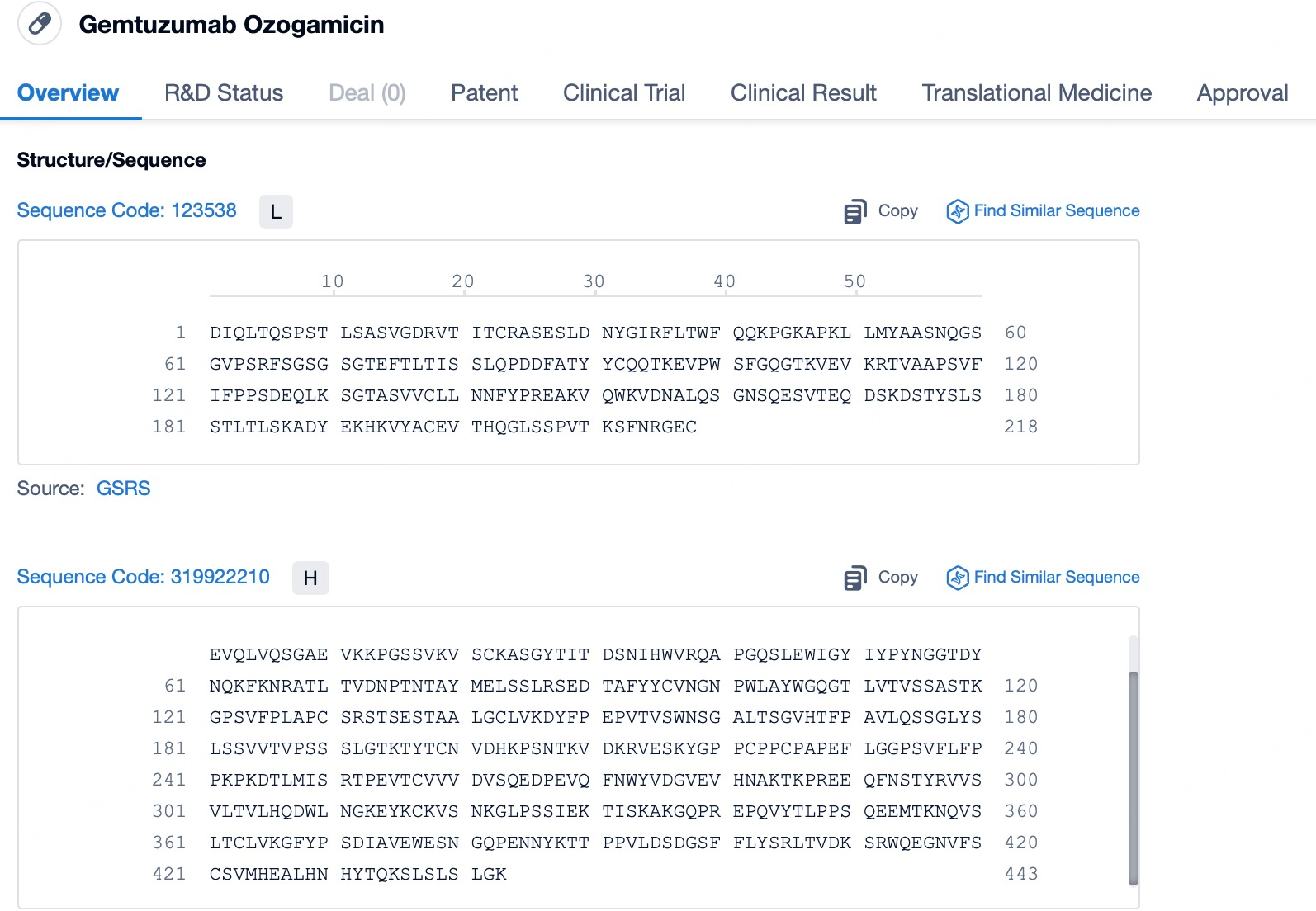
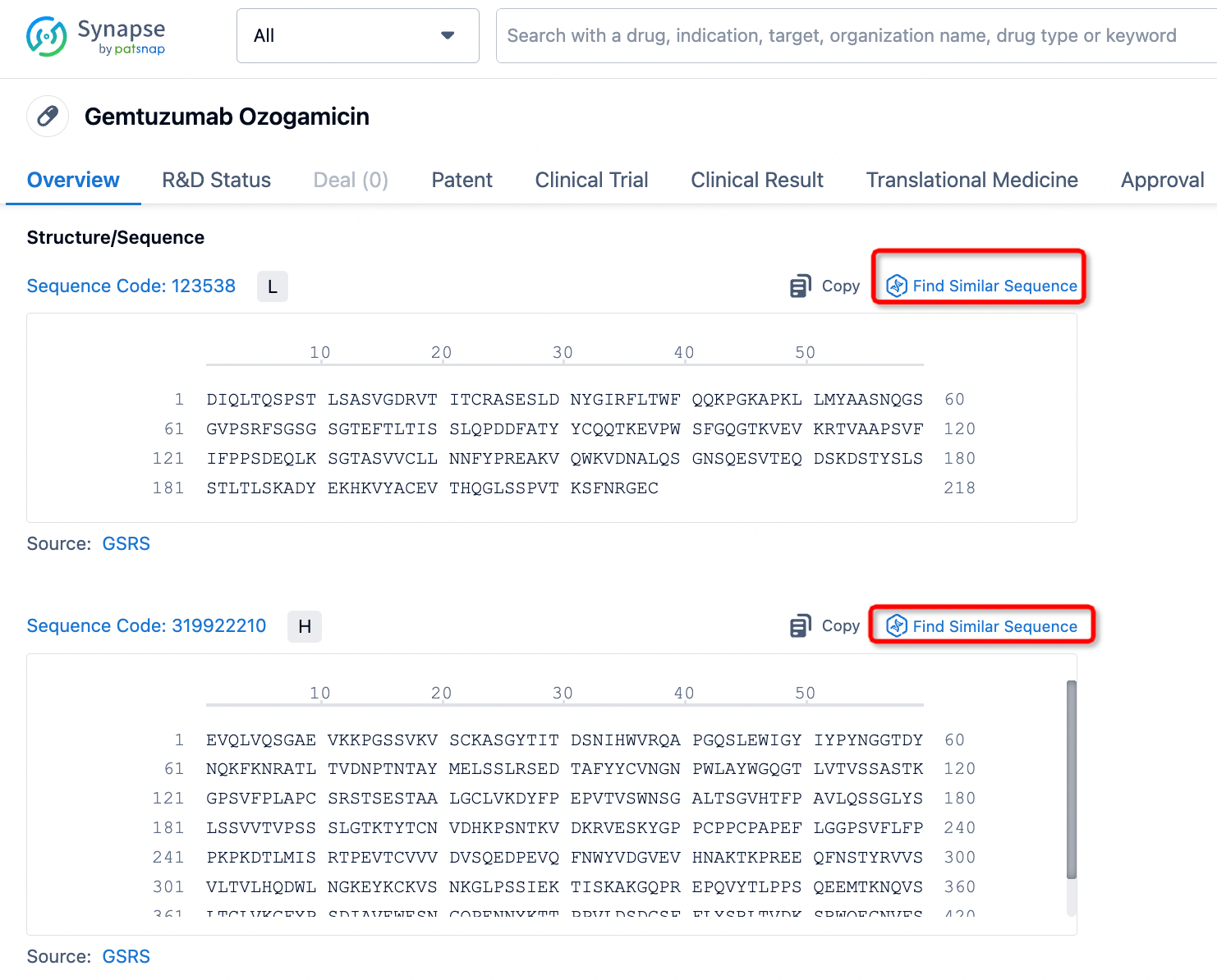
The details page also lists the complete sequence information of the antibody part. By clicking on "Find Similar Sequence", you can be redirected to PatSnap Bio to search for similar sequences of the antibody.
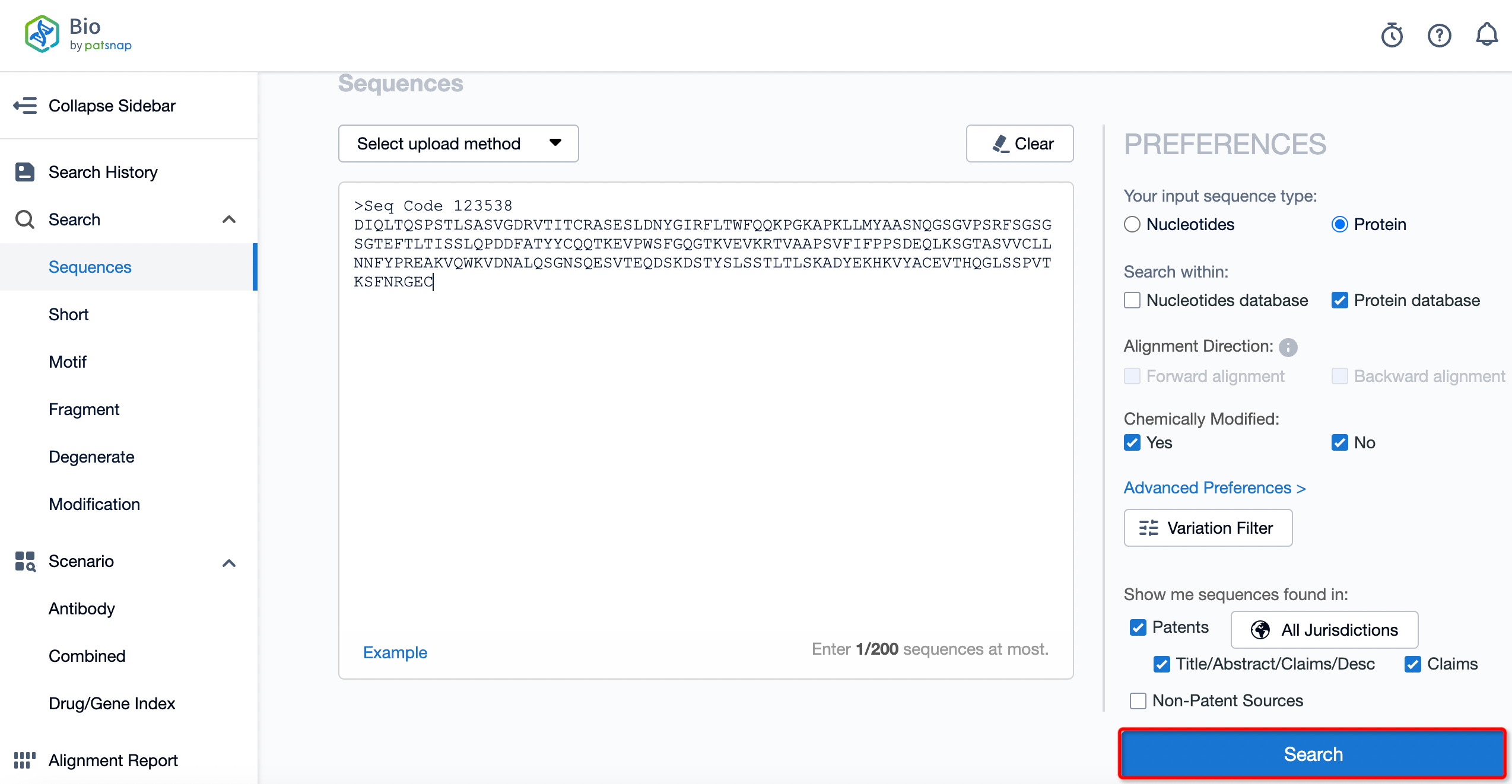
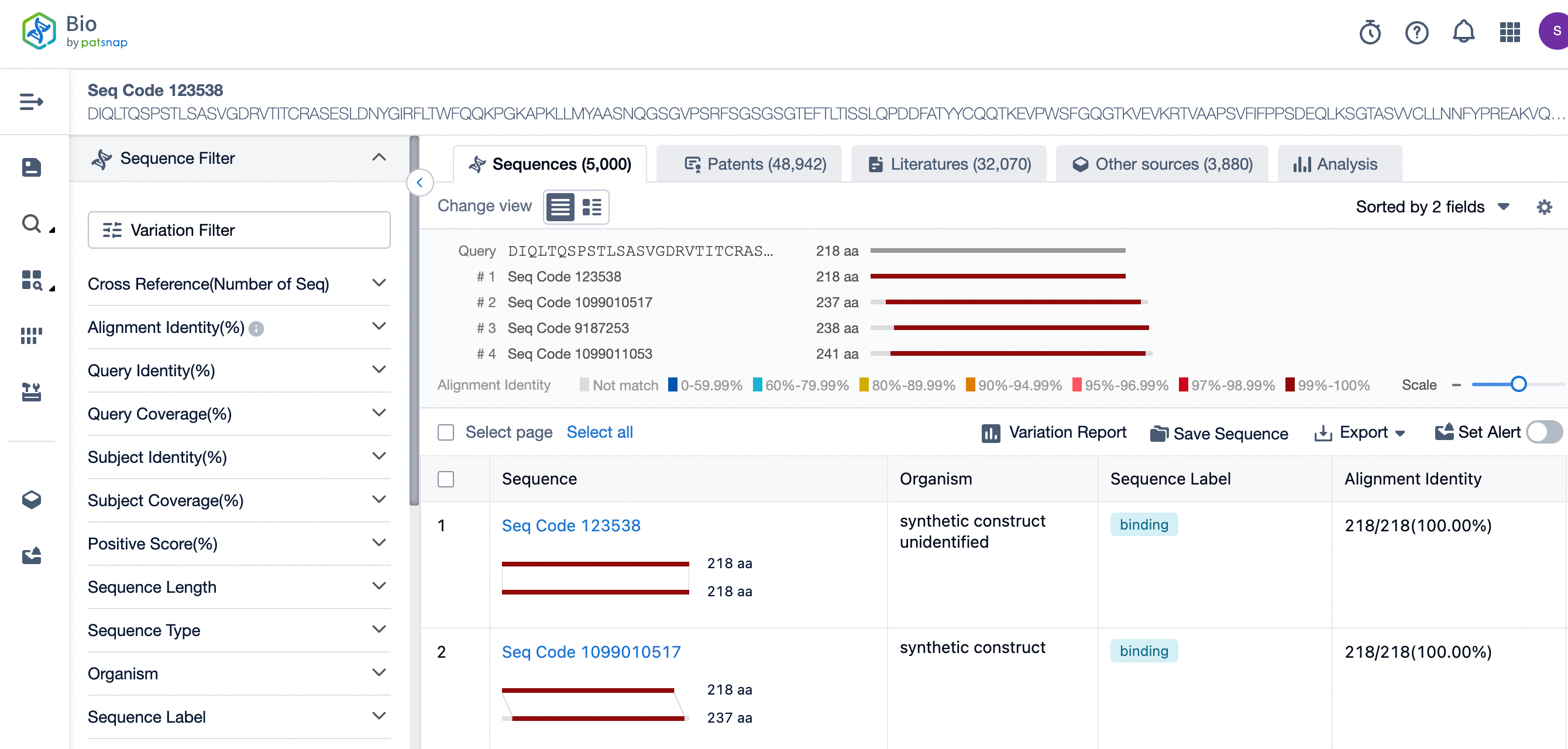
Clicking on the sequence name will provide you with all the basic information of that sequence.
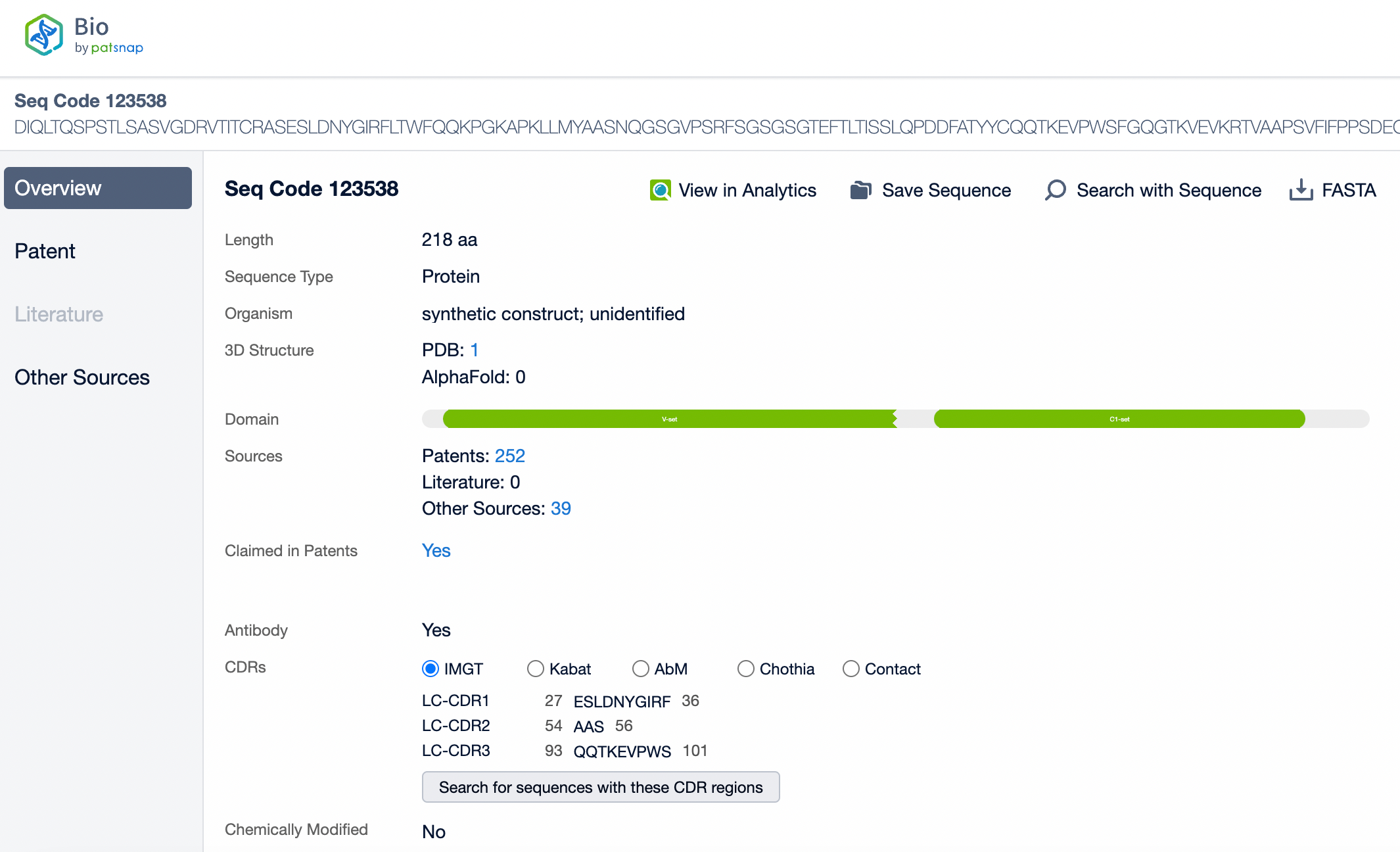
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.