Innovent Announces Success of Phase 2 Trial for Picankibart (IBI112) in Chinese Ulcerative Colitis Patients
Innovent Biologics, Inc.(HKEX: 01801), a leading biopharmaceutical firm focused on the development, production, and commercialization of top-quality medications for oncology, autoimmune disorders, cardiovascular conditions, metabolic issues, ophthalmic diseases, and other significant ailments, has announced that the primary endpoint of the 12-week induction phase was achieved in a multicenter, randomized, double-blind, placebo-controlled phase 2 clinical trial (ClinicalTrials.gov, NCT05377580) evaluating picankibart (R&D code: IBI112), a recombinant anti-interleukin 23p19 subunit (IL-23p19) antibody injection, in Chinese patients suffering from moderately to severely active ulcerative colitis (UC).
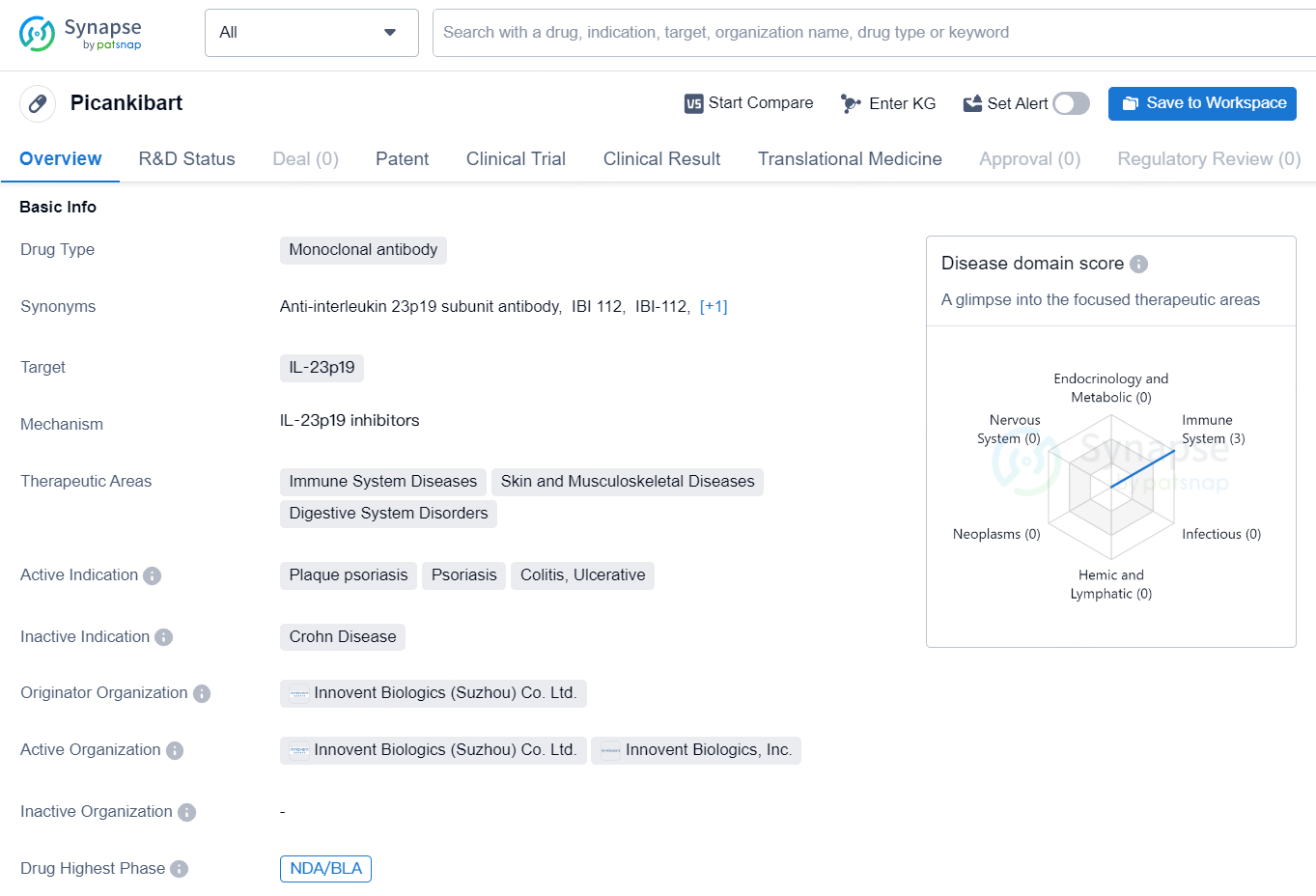
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This research aims to assess the effectiveness and safety of picankibart in treating moderately to severely active ulcerative colitis (with a modified Mayo score ranging from 4 to 9 and an endoscopic subscore of ≥2), involving both induction and maintenance phases. A total of 150 participants were recruited and randomized in a 1:1:1 scheme to receive either an intravenous placebo, picankibart 200 mg, or picankibart 600 mg at baseline, week 4, and week 8 during the induction phase. For the maintenance phase, subjects received subcutaneous picankibart 200 mg injections every 4 or 8 weeks.
The primary outcome measure was the percentage of participants reaching clinical remission at week 12 (according to modified Mayo score, characterized by a rectal bleeding subscore of 0, a stool frequency subscore of ≤1, and an endoscopic subscore of ≤1). Secondary outcomes included the proportion of participants demonstrating clinical response, symptomatic remission, endoscopic remission, or histologic-endoscopic mucosal remission in comparison to the placebo group.
Results showed that both primary and secondary outcomes were achieved: the rate of clinical remission was substantially greater in the picankibart 200 mg group (20.0%) and the 600 mg group (14.0%) compared to the placebo (2.0%; p < 0.05). Clinical response rates for the picankibart 200 mg and 600 mg groups were 54.0% and 68.0%, respectively, significantly outpacing the placebo group (22.0%; p < 0.001). Furthermore, a higher proportion of subjects in both picankibart doses achieved symptomatic remission, endoscopic remission, or histologic-endoscopic mucosal remission compared to the placebo group.
The safety profile of picankibart was found to be good and comparable to previous studies involving other IL-23 class drugs, with no new safety concerns identified. The maintenance phase of the study is currently in progress, with the number of subjects attaining clinical remission, clinical response, symptomatic remission, endoscopic remission, or histologic-endoscopic mucosal remission continuing to rise since the induction phase. Comprehensive data will be analyzed and shared at upcoming academic conferences or in clinical publications.
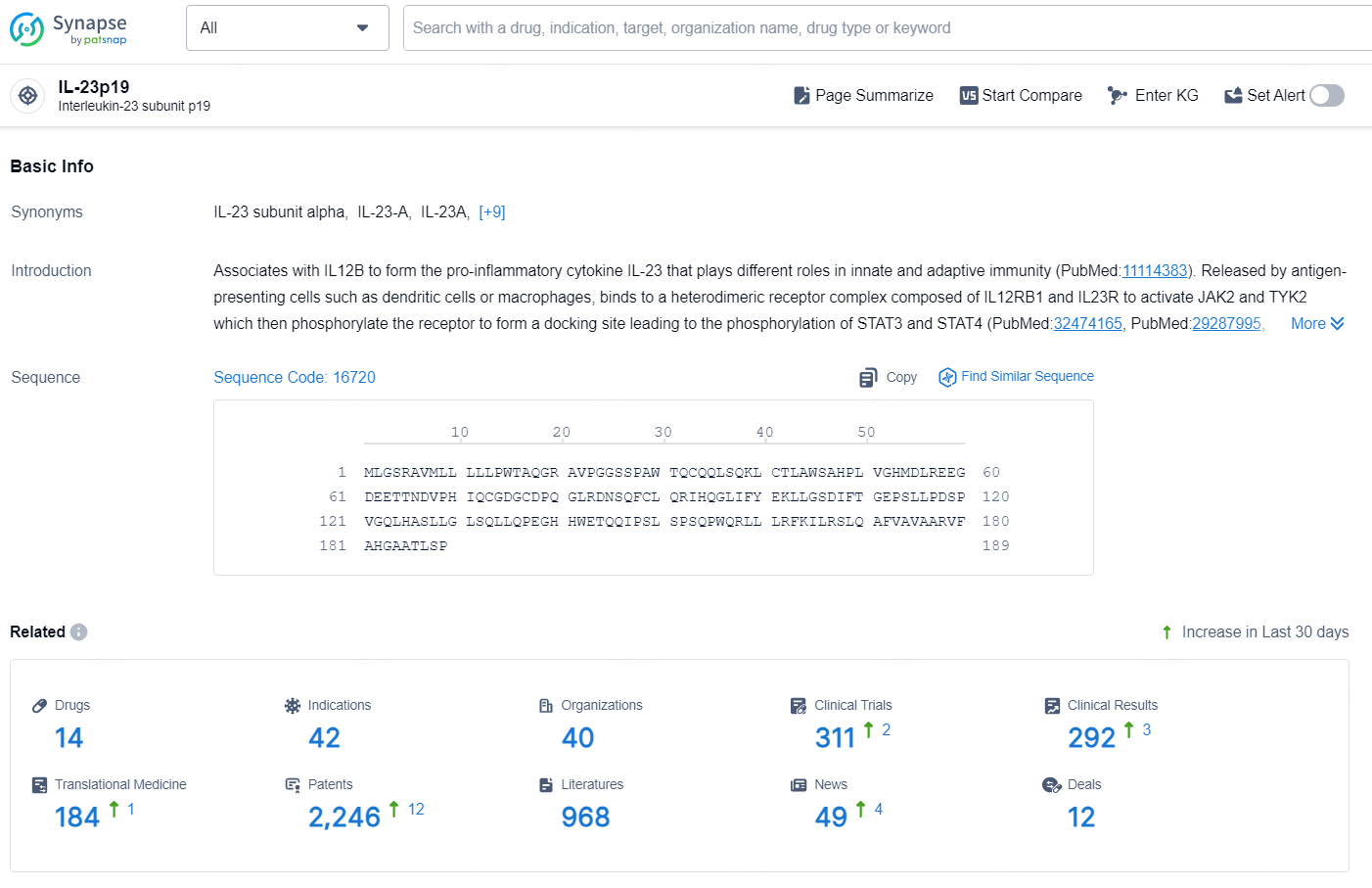
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 18, 2024, there are 14 investigational drugs for the IL-23p19 targets, including 42 indications, 40 R&D institutions involved, with related clinical trials reaching 311, and as many as 2246 patents.
Picankibart is a monoclonal antibody drug that targets IL-23p19 and is developed by Innovent Biologics (Suzhou) Co. Ltd. The drug is primarily intended to treat immune system diseases, skin and musculoskeletal diseases, and digestive system disorders. Specific therapeutic areas include plaque psoriasis, psoriasis, colitis, and ulcerative conditions.