Dosing Begins in IgGenix’s Phase 1 “ACCELERATE Peanut” Trial for Peanut Allergies
IgGenix, Inc., a biotechnology firm in the clinical development phase focused on pioneering treatments for immune-mediated disorders, has announced that the first participant has been dosed in its ACCELERATE Peanut Phase 1 clinical trial. This study is intended to assess IGNX001, a new therapeutic option created through IgGenix's exclusive SEQ SIFTER platform, for individuals suffering from peanut allergy. IGNX001 is engineered to neutralize the key clinical peanut allergens and epitopes, offering a fresh strategy to address peanut allergies by interrupting the allergic response.
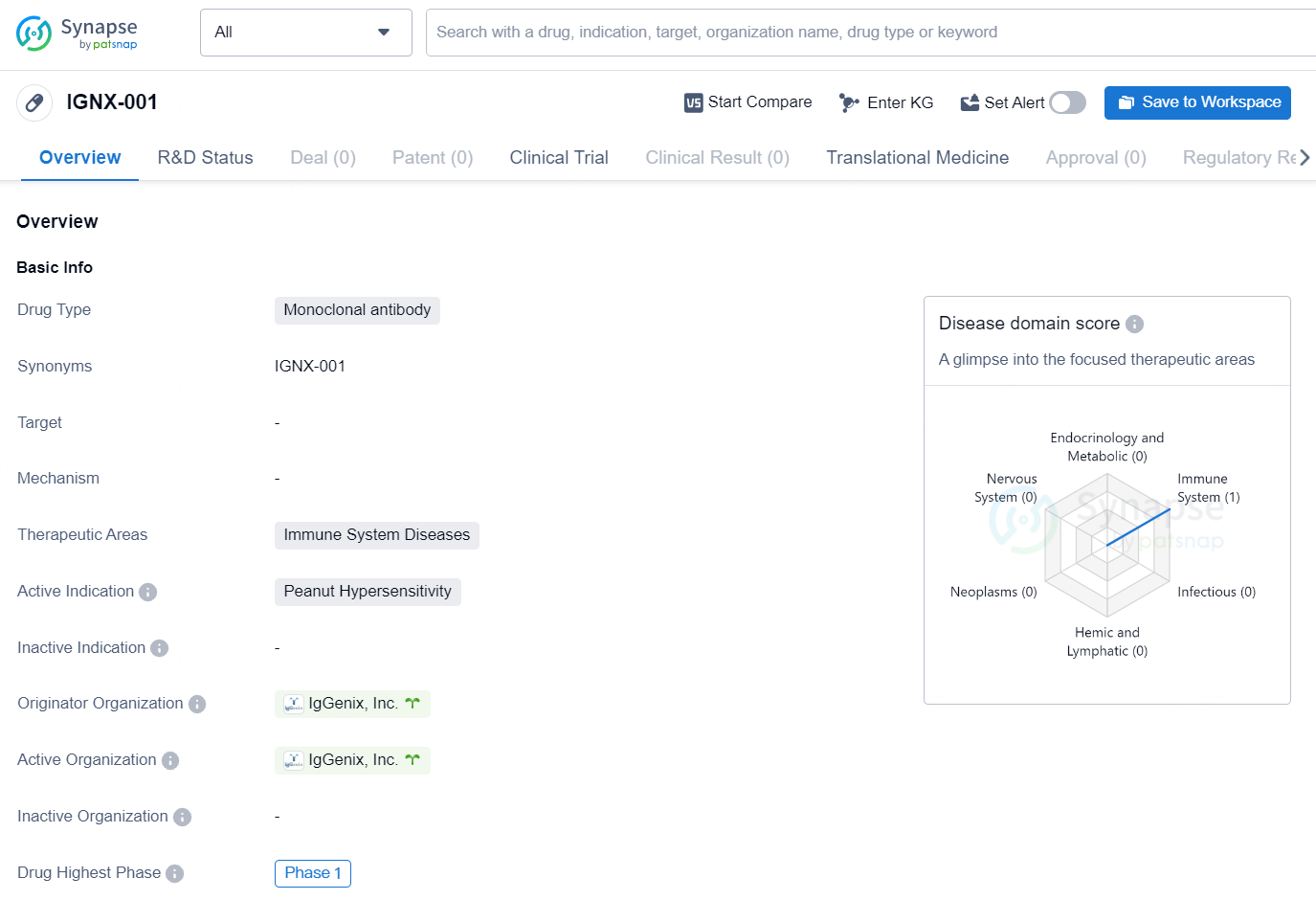
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are excited to announce the launch of our ACCELERATE Peanut Phase 1 clinical trial and the administration of the first dose to a participant," stated Jessica Grossman MD, CEO of IgGenix. "The compound IGNX001 is the result of extensive research and development efforts, and reaching this milestone is a crucial step in confirming our mechanism of action for treating allergies. Preclinical studies indicate that IGNX001 has the potential to offer significant benefits for patients with peanut allergies, especially given its anticipated quick onset, improved safety profile, and a less frequent subcutaneous injection regimen. We are eager to share topline results in mid-2025 and to advance IGNX001 through the clinical process."
The ACCELERATE Peanut Phase 1 trial is a randomized, multi-center, double-blind, placebo-controlled study focusing on a single ascending dose to evaluate the safety, tolerability, and potential proof of mechanism of IGNX001 among individuals allergic to peanuts. The study is aimed at adults and older adolescents (ages 15 to 18), with plans to enroll approximately 24 participants diagnosed with peanut allergies.
"For those with peanut allergies, there is always the risk of severe, potentially fatal allergic reactions which can create significant physical, social, and emotional challenges. The innovative mechanism of action of IGNX001 presents an exciting opportunity, and I am optimistic that it could transform the management of peanut allergies by lessening the risk of anaphylaxis," remarked Dr. Andrew Carr, DSc MD MBBS FRACP FRCPA, Senior Staff Specialist in Immunology and HIV at St Vincent’s Hospital in Sydney and Professor of Medicine at UNSW Sydney.
This Phase 1 trial is the first program in the pipeline at IgGenix. If successful, IGNX001 could not only offer a promising treatment for peanut allergies but also open avenues for applying IgGenix’s technology to other food allergies and allergic conditions.
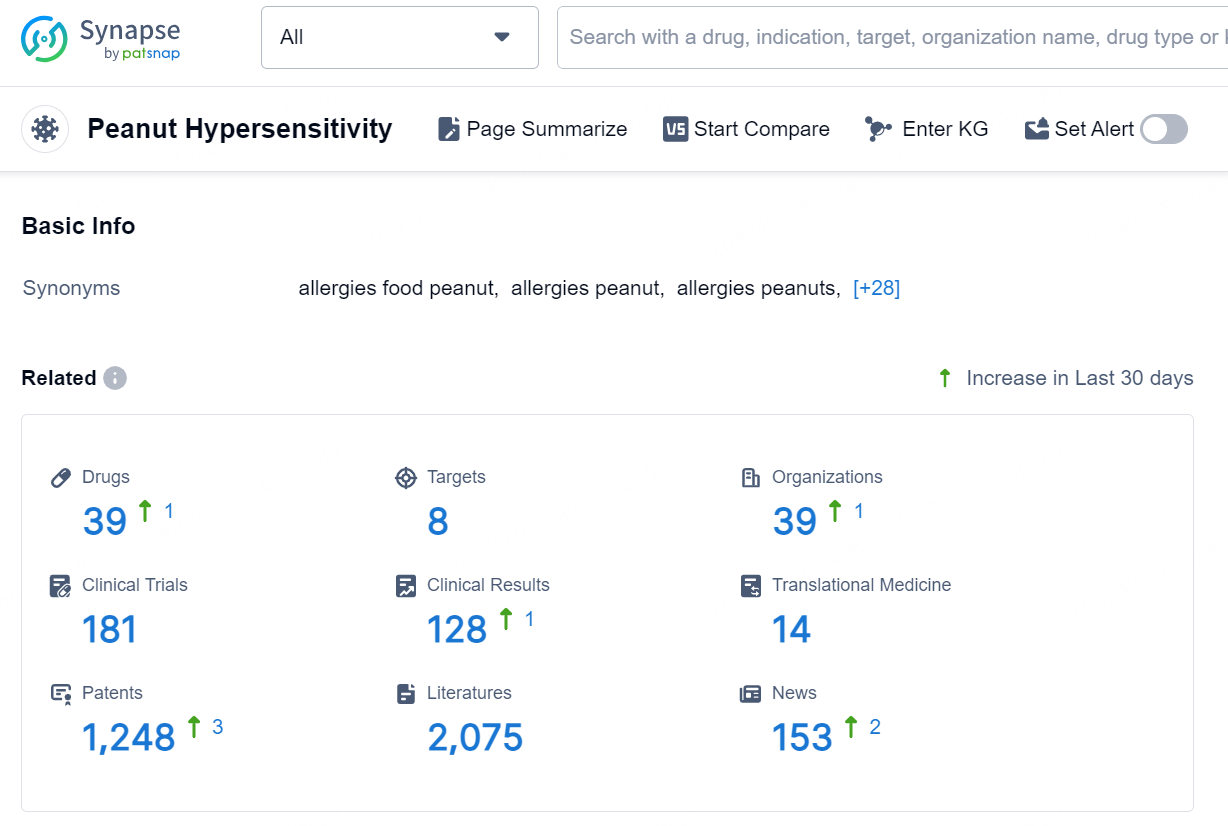
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 18, 2024, there are 39 investigational drugs for the peanut hypersensitivity, including 8 targets, 39 R&D institutions involved, with related clinical trials reaching 181, and as many as 1248 patents.
IGNX-001 is a monoclonal antibody drug developed by IgGenix, Inc. The drug falls under the therapeutic area of immune system diseases, with its active indication being peanut hypersensitivity. As of the latest information available, IGNX-001 has reached the highest phase of development at a global level, which is Phase 1.