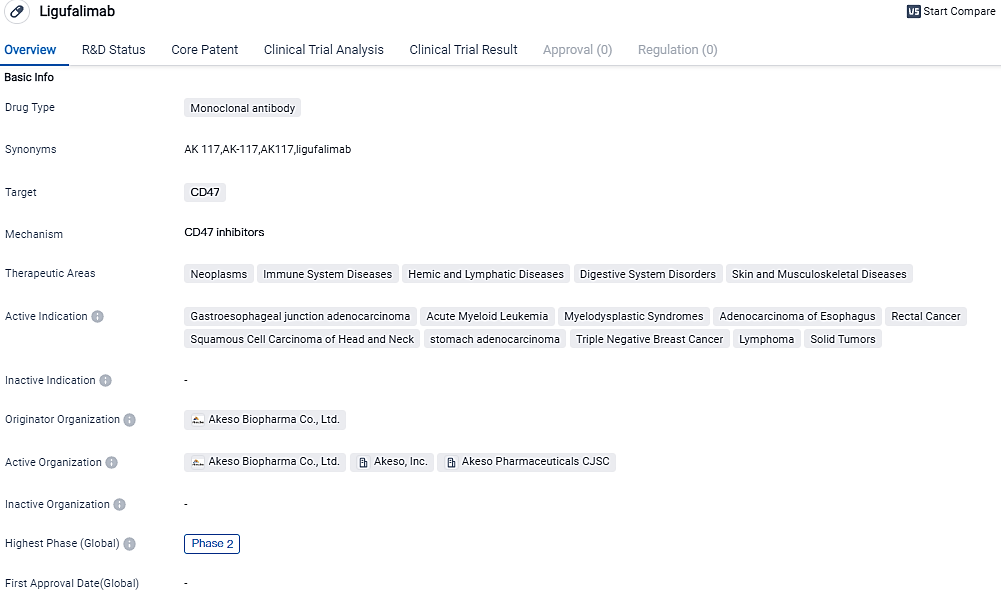
Akeso announced FDA's approval of their IND for AK117/Azacitidine combo treatment for Myelodysplastic Syndromes
Akeso, Inc. has made it known that their IND application for AK117, which pairs a revolutionary CD47 monoclonal antibody with azacitidine, has been approved by the U.S. FDA. This treatment is intended for patients who have just been diagnosed with high-risk myelodysplastic syndromes. The imminent research, identified as a double-blind, randomized, worldwide multicenter Phase II study, will be carried out in the U.S.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Akeso has engaged in thorough scientific exchanges and conversations with the FDA, based on AK117's impressive track record of efficacy and safety when used in conjunction with azacitidine in the treatment of newly diagnosed higher-risk MDS patients, as proven in prior research. Given the global demand from MDS patients, Akeso is confident that this study will accelerate the worldwide advancement of AK117.
"By granting us the luxury of time and space, we will steadfastly pursue a rigorous global approach in the investigation and progress of our groundbreaking therapies. For the good of a broader patient population, we will harness global resources to augment the success rate of AK117," declared Dr. Michelle Xia, who is the founder, Chairwoman, CEO, and President of Akeso.
Controlling anemia, a hallmark symptom of MDS, and limiting the necessity for blood transfusion are integral components of disease management. Unlike other anti-CD47 antibody drugs which reportedly exacerbate anemia in MDS patients by inducing RBC agglutination, AK117 eradicates RBC agglutination while simultaneously preserving full effectiveness of CD47 blockade on cancer cells. This leads to its superior antitumor efficacy and safety profile of AK117.
Previous research of AK117 used with azacitidine revealed encouraging results in MDS patients who were newly diagnosed with higher-risk. AK117 lessened anemia and the demands for transfusion in MDS patients, while revealing an outstanding safety profile and superb efficacy. Such outcomes position AK117 as an optimistic treatment choice for MDS patients across the globe.
It's worth noting that Akeso is unyielding in its progress of AK117 as a therapeutic aid used with various other agents like PD-1/CTLA-4 and PD-1/VEGF, in the treatment of numerous hematologic malignancies and solid tumors.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
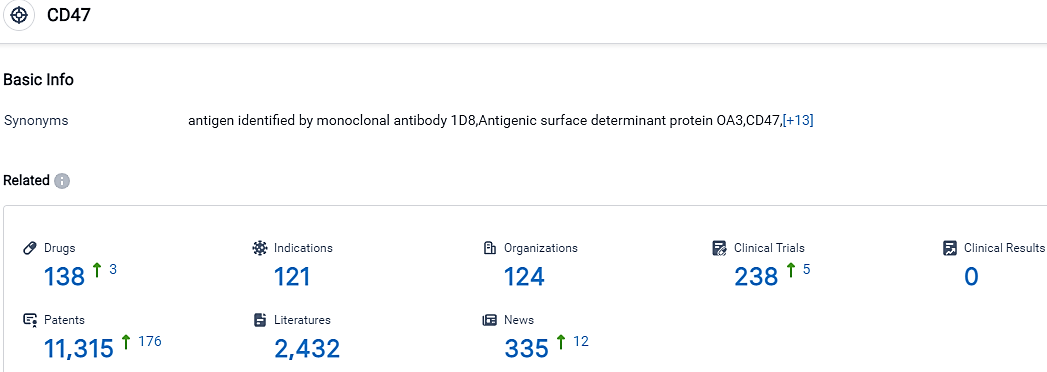
According to the data provided by the Synapse Database, As of October 5, 2023, there are 138 investigational drugs for the CD47 target, including 121 indications,124 R&D institutions involved, with related clinical trials reaching 238,and as many as 11315 patents.
AK117, a unique product of Akeso, represents the future of humanized lgG4 anti-CD47 antibodies and it does not induce hemagglutination. By attaching itself to CD47 proteins found on cancer cells, AK117 interferes with the interaction between CD47 and SIRPα. This increases the capacity of phagocytes to consume cancer cells, thus staving off tumor growth. Ligufalimab getting to this point implies encouraging outcomes from the previous stages of its trials.