Alpha Cognition’s Oral Therapy ZUNVEYL® Receives FDA Approval to Treat Alzheimer’s Disease
Alpha Cognition, a biopharmaceutical firm dedicated to creating new treatments for severe neurodegenerative diseases, has reported that the U.S. Food and Drug Administration has given the green light to ZUNVEYL® (benzgalantamine), formerly referred to as ALPHA-1062, for addressing mild-to-moderate Alzheimer’s disease.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Alzheimer’s disease, a chronic progressive disorder of the brain, gradually diminishes memory, cognitive abilities, and eventually the capacity to perform simple tasks such as conversing. As the most prevalent type of dementia, it impacts nearly 7 million people and is the primary cause of admissions to nursing homes and related mortalities, with 70% of nursing home residents affected by AD.
ZUNVEYL is a new oral therapy with a dual mechanism of action aimed at eliminating drug absorption in the gastrointestinal tract, potentially addressing tolerability problems observed with leading AD medications, while retaining the efficacy and long-term benefits of galantamine. Tolerability is crucial for adherence to treatment regimens; data indicates that 55% of AD patients stop their medication within a year, primarily due to gastrointestinal side effects and insomnia. Discontinuing medication can pose risks for patients and result in dissatisfaction and increased responsibility among nursing home staff, healthcare providers, and caregivers.
ZUNVEYL, a prodrug of the AD treatment galantamine and an acetylcholinesterase inhibitor, is believed to function therapeutically by preventing the breakdown of acetylcholine, a critical neurotransmitter involved in memory, motivation, and attention. It also acts as an allosteric potentiator of α-7 nicotinic acetylcholine and α4β2 receptors.
This mechanism encourages the release of acetylcholine from presynaptic neurons, adding clinical value to its dual action mode. ZUNVEYL addresses AD symptoms, offering patients sustained enhancements in cognitive and overall functions and the ability to carry out daily activities hampered by AD. Galantamine, which received FDA approval in 2001, has a substantial and favorable data set regarding long-term outcomes, showing interaction with various brain receptors, anti-inflammatory properties, and associations with improved memory, attention, and a notably lower mortality risk.
Due to its prodrug features, ZUNVEYL efficiently converts into the active form of galantamine once it passes through the GI tract, thus achieving galantamine's therapeutic benefits. It was specifically designed to avoid drug absorption in the GI tract, potentially solving tolerability issues and presents a CNS safety profile without incidences of insomnia. Further information on ZUNVEYL’s critical clinical trials is available in this press release and the ZUNVEYL prescribing information.
“The approval of ZUNVEYL marks a significant milestone in combating Alzheimer’s disease as it is only the second oral treatment for AD approved in over ten years. ZUNVEYL meets an essential need for a tolerable and effective treatment that can potentially improve patients’ quality of life with better long-term outcomes,” stated Alpha Cognition Chief Executive Officer Michael McFadden. “We are thrilled, as this signifies a major advancement in Alzheimer’s treatment, offering hope to millions of patients, families, and caregivers affected by this debilitating disease.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
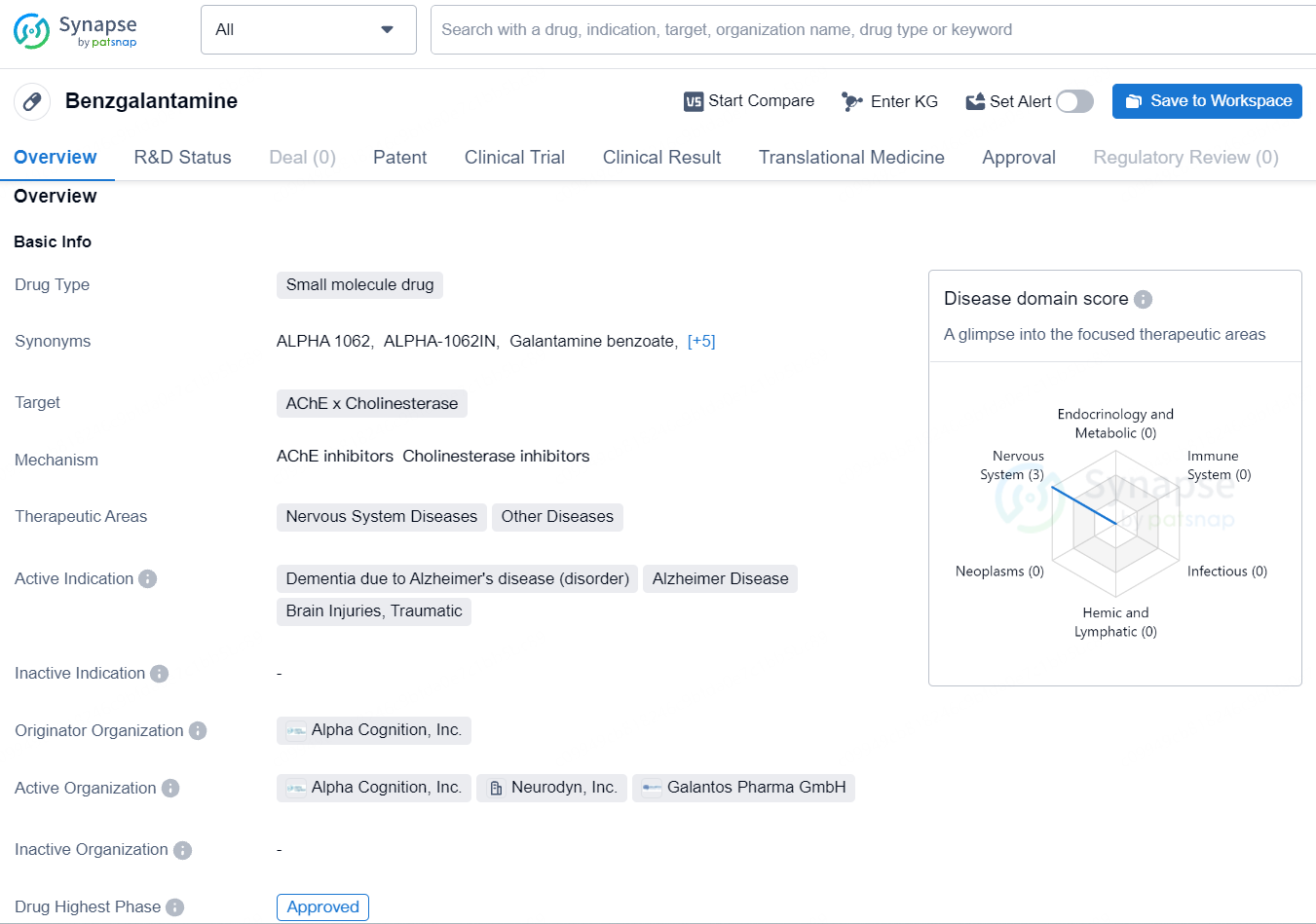
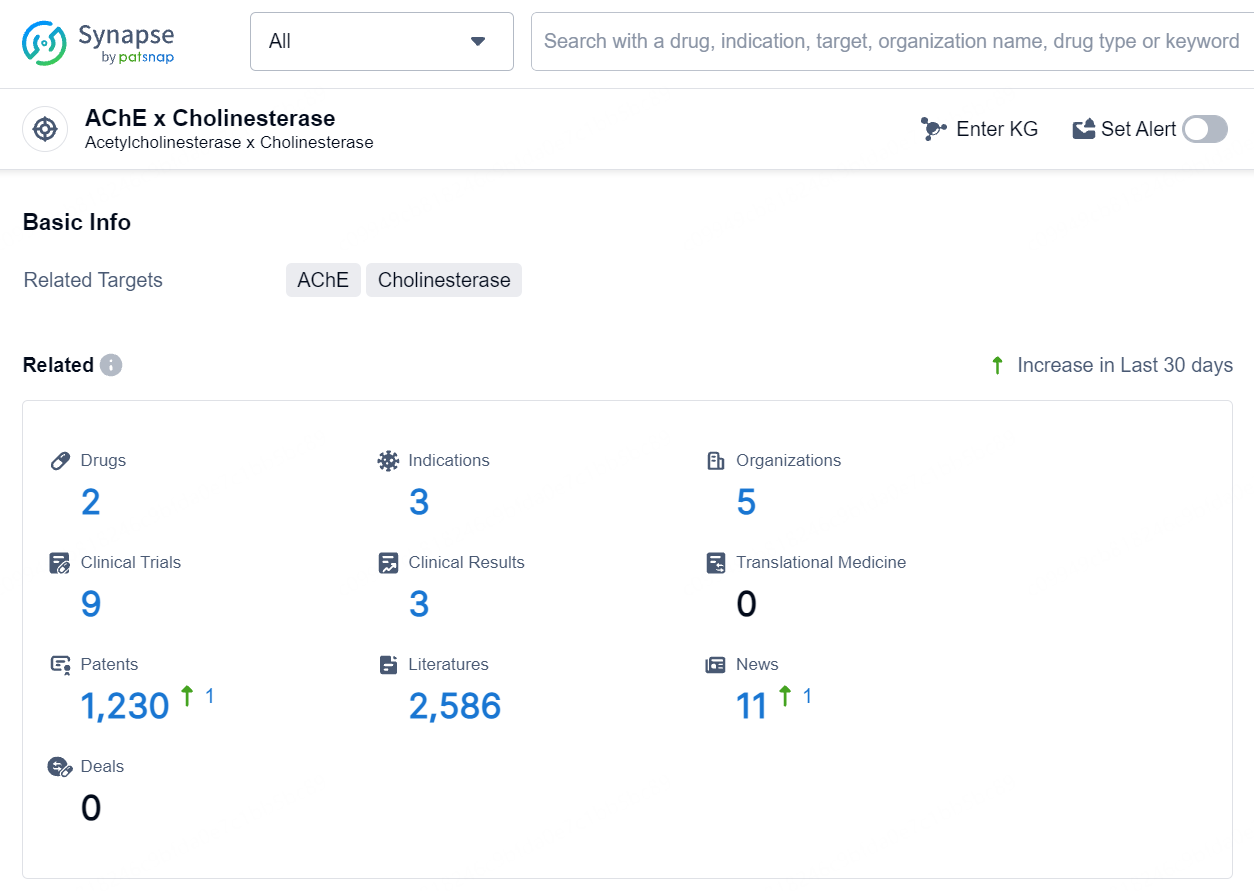
According to the data provided by the Synapse Database, As of August 1, 2024, there are 2 investigational drugs for the AChE (Acetylcholinesterase) and cholinesterase target, including 3 indications, 5 R&D institutions involved, with related clinical trials reaching 9, and as many as 1230 patents.
Benzgalantamine targets AChE (Acetylcholinesterase) and cholinesterase and is primarily used in the therapeutic areas of Nervous System Diseases and Other Diseases. The active indications for Benzgalantamine include the treatment of Dementia due to Alzheimer's disease, Alzheimer Disease, and Brain Injuries, Traumatic. Benzgalantamine represents a novel therapeutic option for managing conditions such as Alzheimer's disease, traumatic brain injuries, and other related neurological disorders. Its approval and targeted indications position it as a valuable asset in the pharmaceutical industry, with the potential to positively impact patient outcomes and contribute to advancements in the treatment of nervous system diseases.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!