Is VOWST approved by the FDA?
Yes, VOWST is FDA approved. The FDA approved VOWST on April 26, 2023, for the prevention of recurrence of Clostridioides difficile infection (CDI) in adults following antibacterial treatment for recurrent CDI.
What is VOWST?
VOWST is a prescription medicine used in adults to prevent the recurrence of CDI after completing antibiotic treatment for C. difficile infection. It is an oral microbiome therapeutic consisting of live bacteria, manufactured from human fecal matter sourced from qualified donors. VOWST works by restoring the gut microbiome to a balanced state that resists C. difficile colonization and growth.
Usage and Indications
VOWST is specifically used for:
- Preventing the recurrence of CDI in individuals aged 18 and older after antibacterial treatment for recurrent CDI.
How to Take VOWST
- Pre-treatment Preparation:
- 2 to 4 days before the first dose of VOWST, finish the antibiotic treatment for CDI.
- On the day before and at least 8 hours prior to the first dose, drink 10 oz (296 mL) of magnesium citrate laxative.
- Do not eat or drink, except for small amounts of water, for at least 8 hours before taking the first dose.
- Dosage:
- Take 4 VOWST capsules by mouth on an empty stomach before the first meal of the day.
- Continue taking 4 capsules once daily for two more consecutive days.
- Swallow each capsule whole without crushing, chewing, or breaking them.
Side Effects
Common Side Effects
- Bloated abdomen
- Fatigue
- Constipation
- Chills
- Diarrhea
Severe Side Effects
If you experience any severe side effects, contact your healthcare provider immediately.
Warnings and Precautions
- Pregnancy and Breastfeeding:
- It is not known if VOWST is safe during pregnancy or if it is excreted in breastmilk. Discuss with your healthcare provider if you are pregnant, planning to become pregnant, or breastfeeding.
- Drug Interactions:
- Do not take VOWST simultaneously with antibiotics, as they can interfere with the live bacteria in VOWST.
- Medical Conditions:
- Inform your healthcare provider if you have kidney disease or any other medical conditions.
Storage Instructions
- Keep the bottle of capsules either in the refrigerator or at room temperature (36 to 77°F, 2 to 25°C). Do not freeze.
- Do not use VOWST past the expiration date on the carton and bottle.
- Keep all medicines out of the reach of children and pets.
Conclusion
VOWST, approved by the FDA on April 26, 2023, offers a novel approach to preventing the recurrence of CDI in adults following antibacterial treatment. By restoring the gut microbiome, VOWST helps maintain a balanced state that resists C. difficile colonization and growth. Always follow your healthcare provider's instructions when using VOWST and report any side effects or concerns promptly.
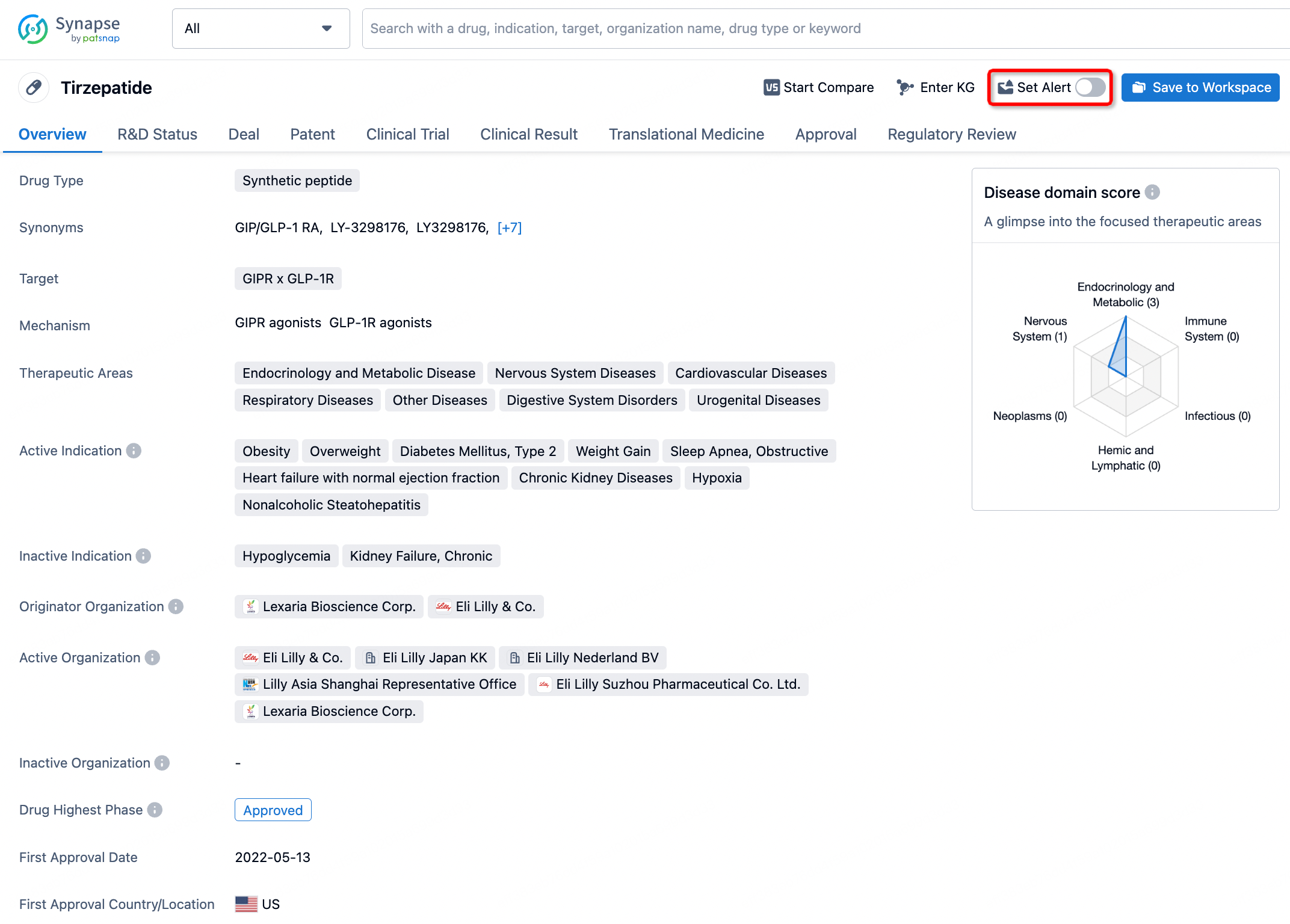
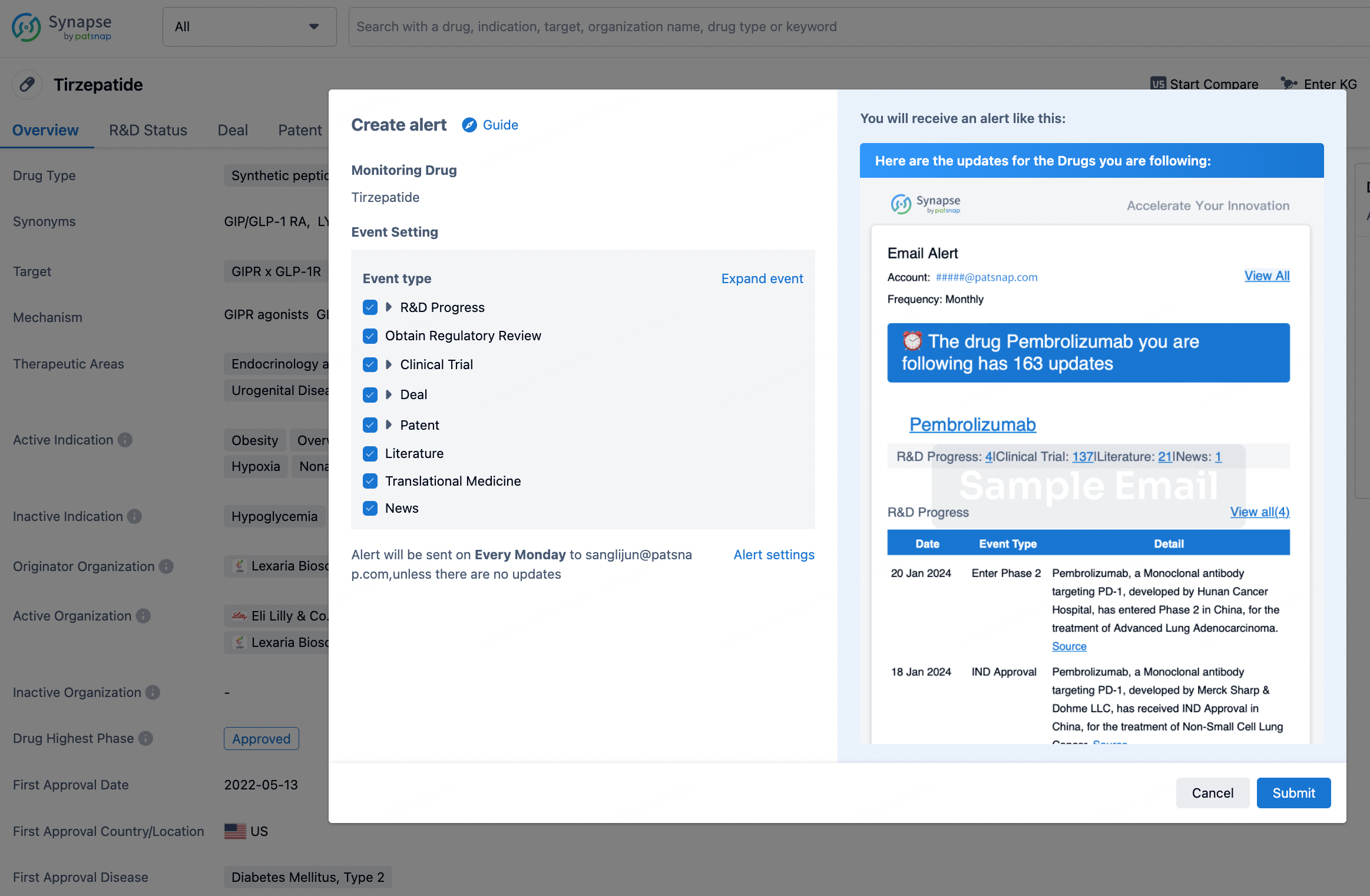
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!